Just a Few Things 2012
... Boiling occurs when the vapor pressure = atmospheric pressure “normal boiling point” = boiling point at 1 atm pressure (101.3 kPa) ...
... Boiling occurs when the vapor pressure = atmospheric pressure “normal boiling point” = boiling point at 1 atm pressure (101.3 kPa) ...
3 - Zheng Research Group
... then, it has 36.4 g Mn, 21.2 g S, and 42.4 g O; Mn: 36.4 g / 54.9 g/mol = 0.663 mol Mn; S: 21.2 g / 32.1 g/mol = 0.660 mol S; O: 42.4 g / 16.0 g/mol = 2.65 mol O. therefore: divide the smallest number (0.660), the formula is: MnSO4. ...
... then, it has 36.4 g Mn, 21.2 g S, and 42.4 g O; Mn: 36.4 g / 54.9 g/mol = 0.663 mol Mn; S: 21.2 g / 32.1 g/mol = 0.660 mol S; O: 42.4 g / 16.0 g/mol = 2.65 mol O. therefore: divide the smallest number (0.660), the formula is: MnSO4. ...
View attached file
... Daniel Segal - Research 'Conformational diseases' are diseases caused by misfolding of a protein, often as a result of a missense mutation that does not necessarily disrupt the active site of the protein. As a result, the protein may lose its function, and often the misfolded monomers self-assemble ...
... Daniel Segal - Research 'Conformational diseases' are diseases caused by misfolding of a protein, often as a result of a missense mutation that does not necessarily disrupt the active site of the protein. As a result, the protein may lose its function, and often the misfolded monomers self-assemble ...
Lecture 1
... Calculations show that proteins are NOT very stable molecules Net stabilization of ~0.4 kJ mol-1 for each amino acid ...
... Calculations show that proteins are NOT very stable molecules Net stabilization of ~0.4 kJ mol-1 for each amino acid ...
Corn Gluten Meal - International Feed
... It is also valued in pet food for its high protein digestibility. The product is golden yellow in color; and fine granular in its physical appearance. Due to its high protein content, CGM is mostly used as a source of protein as a potential alternative to other plant or animal-based proteins, such a ...
... It is also valued in pet food for its high protein digestibility. The product is golden yellow in color; and fine granular in its physical appearance. Due to its high protein content, CGM is mostly used as a source of protein as a potential alternative to other plant or animal-based proteins, such a ...
Examples of Biomaterials
... associated with protein adhesion to hydrophobic surfaces • Attraction due to long-range van der Waals forces, as well as specific and hydrophobic interactions, and the electrostatic double layer (all short-range) • Repulsion due to steric and osmotic factors (short range) • Proteins will stick if Ub ...
... associated with protein adhesion to hydrophobic surfaces • Attraction due to long-range van der Waals forces, as well as specific and hydrophobic interactions, and the electrostatic double layer (all short-range) • Repulsion due to steric and osmotic factors (short range) • Proteins will stick if Ub ...
Final Preparation
... ____C3H8 + ____O2 ____CO2 + ____H2O A. 5.00 B. 10.0 C. 15.0 D. 25.0 69. What is mutarotation? A. The conversion of a D-monosaccharide into an L-monosaccharide. B. The conversion of α-D-galactose into β-D-galactose C. The conversion of a pyranose into a furanose. D. The conversion of an aldose into a ...
... ____C3H8 + ____O2 ____CO2 + ____H2O A. 5.00 B. 10.0 C. 15.0 D. 25.0 69. What is mutarotation? A. The conversion of a D-monosaccharide into an L-monosaccharide. B. The conversion of α-D-galactose into β-D-galactose C. The conversion of a pyranose into a furanose. D. The conversion of an aldose into a ...
MCB100A/CHEM130A In-Section Quiz #2 (Aathavan Karunakaran)
... ordering briefly (atmost 2-3 sentences) (3) ...
... ordering briefly (atmost 2-3 sentences) (3) ...
Transport Across Cell Membrane (Powerpoint)
... The Importance of Cell surface to Volume Ratio The rate at which these substances cross this membrane depends of the surface area. The surface area to volume ratio of a cell is therefore very important. 1×1×1 cm cube has a surface area of 6×1×1 cm, or 6 cm² with a volume of 1 cm³. 2×2×2 cm cub ...
... The Importance of Cell surface to Volume Ratio The rate at which these substances cross this membrane depends of the surface area. The surface area to volume ratio of a cell is therefore very important. 1×1×1 cm cube has a surface area of 6×1×1 cm, or 6 cm² with a volume of 1 cm³. 2×2×2 cm cub ...
Chromatography - Union College
... dextran – (x-linked to different pore sizes) supplied as dry beads = Sephadex, or agarose – H-bonded, so concentration determines pore size – used for large proteins and DNA (Sepharose or Biogel) or polyacrylamide (xlinked) ...
... dextran – (x-linked to different pore sizes) supplied as dry beads = Sephadex, or agarose – H-bonded, so concentration determines pore size – used for large proteins and DNA (Sepharose or Biogel) or polyacrylamide (xlinked) ...
2. Covalent network
... constant R. R=.0821 (L*atm)/(mol*k) It can be used to solve for pressure, number of moles, volume, or temberature when all other variables are held constant. At STP (0C and 1atm), the molar volume of an ideal gas is 22.42 L. (Constant) Dalton’s Law of Partial Pressures: Ptotal=ntotal(RT/V) Used to ...
... constant R. R=.0821 (L*atm)/(mol*k) It can be used to solve for pressure, number of moles, volume, or temberature when all other variables are held constant. At STP (0C and 1atm), the molar volume of an ideal gas is 22.42 L. (Constant) Dalton’s Law of Partial Pressures: Ptotal=ntotal(RT/V) Used to ...
Fluorine-Adding Bacteria May Transform Natural Product Medicines
... unwanted small molecules in the body, as well as diagnostics that can find similarly small targets. But more than that, Kuhlman says, the work restores protein engineers’ confidence that they can solve one of their greatest challenges. “It’s a very powerful example that in some cases ...
... unwanted small molecules in the body, as well as diagnostics that can find similarly small targets. But more than that, Kuhlman says, the work restores protein engineers’ confidence that they can solve one of their greatest challenges. “It’s a very powerful example that in some cases ...
Molecular Dynamics Simulations of the Purple Membrane
... External hydration (larger orange spheres) penetrates into bR up to the Arg82 & Asp96 levels ...
... External hydration (larger orange spheres) penetrates into bR up to the Arg82 & Asp96 levels ...
Molecular size scales in biology
... 3. Lac repressor complex with DNA 4. Hemoglobin 5. Myoglobin 6. Green fluorescent protein (GFP) 7. RNA polymerase • Download a program that allows structure viewing such as RasMol (U. Massachusettes). • Use the measurement tools in these programs to measure the maximal length of these molecules (max ...
... 3. Lac repressor complex with DNA 4. Hemoglobin 5. Myoglobin 6. Green fluorescent protein (GFP) 7. RNA polymerase • Download a program that allows structure viewing such as RasMol (U. Massachusettes). • Use the measurement tools in these programs to measure the maximal length of these molecules (max ...
(p. 522)
... C.Hydrogen molecules and atoms occupy holes within the crystal structure of the metal. D.These substances are useful catalysts. E.These hydrides are stabilized by hydrogen bonding forces. 8. Xenon forms several compounds with oxygen and fluorine. It is the most reactive non-radioactive noble gas bec ...
... C.Hydrogen molecules and atoms occupy holes within the crystal structure of the metal. D.These substances are useful catalysts. E.These hydrides are stabilized by hydrogen bonding forces. 8. Xenon forms several compounds with oxygen and fluorine. It is the most reactive non-radioactive noble gas bec ...
Answers to Mastering Concepts Questions
... 2. Distinguish between nonpolar covalent bonds, polar covalent bonds, and ionic bonds. Nonpolar covalent bonds are bonds in which both atoms exert approximately equal pull on the shared electrons. In a polar covalent bond, one nucleus exerts a stronger pull on the shared electrons than does the othe ...
... 2. Distinguish between nonpolar covalent bonds, polar covalent bonds, and ionic bonds. Nonpolar covalent bonds are bonds in which both atoms exert approximately equal pull on the shared electrons. In a polar covalent bond, one nucleus exerts a stronger pull on the shared electrons than does the othe ...
A Student want to prepare 250mL of .10 M NaCl solution
... – Liters of solution (concentration) – Gas (Ideal gas law) ...
... – Liters of solution (concentration) – Gas (Ideal gas law) ...
defend your answer in 1
... false A covalent bond is likely to be polar when it is between two atoms that are both very strong electron acceptors false If a species genome is divided into 10 chromosomes, that means that all of the genetic information for the species is contained in 5 different double-stranded DNA polymers. tru ...
... false A covalent bond is likely to be polar when it is between two atoms that are both very strong electron acceptors false If a species genome is divided into 10 chromosomes, that means that all of the genetic information for the species is contained in 5 different double-stranded DNA polymers. tru ...
Conformational dynamics of signaling proteins and ion channels
... intercellular domain. Physiologically active preparations of the membrane proteins in detergent were used to define the structural states of the protein. These modifications were quantified and identified using high-resolution mass spectrometry. The differences in the extent of such modifications be ...
... intercellular domain. Physiologically active preparations of the membrane proteins in detergent were used to define the structural states of the protein. These modifications were quantified and identified using high-resolution mass spectrometry. The differences in the extent of such modifications be ...
The Chemistry of Life
... Polar molecules can interact with each other and with ions Whole molecules can be polar or select bonds in a molecule can be polar ...
... Polar molecules can interact with each other and with ions Whole molecules can be polar or select bonds in a molecule can be polar ...
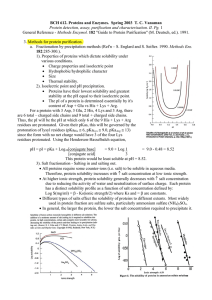
General Reference - Methods Enzymol. 182 "Guide to Protein
... % Native structure Average protein ...
... % Native structure Average protein ...
Membrane & Transport Review
... If the solute concentration in a cell is the same as the solute concentration outside the cell, the cell is in a state of ……. ...
... If the solute concentration in a cell is the same as the solute concentration outside the cell, the cell is in a state of ……. ...
CARBOHYDRATES
... Molecules that contain carbon are called organic compounds. There are over 2 million known organic compounds. ...
... Molecules that contain carbon are called organic compounds. There are over 2 million known organic compounds. ...