Passive and active transport
... Calculate the change in free energy in transporting one gram molecular weight of glucose up a hundred fold gradient from a compartment in which its conc is 0.001 M to a compartment in which conc is 0.1 M at 25 °C. ...
... Calculate the change in free energy in transporting one gram molecular weight of glucose up a hundred fold gradient from a compartment in which its conc is 0.001 M to a compartment in which conc is 0.1 M at 25 °C. ...
Slide 1
... Detergent interactions with Bilayers: last week lecture and presentation. Small Probe Molecule: EPR and Fluorescent Molecules ...
... Detergent interactions with Bilayers: last week lecture and presentation. Small Probe Molecule: EPR and Fluorescent Molecules ...
No Slide Title
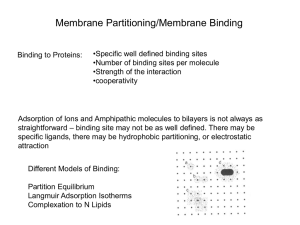
... • Typically, ca. 500 - 1500 Å2 of surface buried upon complex formation by two globular proteins • Epitopes on protein surface thus may have a “hybrid” character, compatible with both a solvent-exposed (‘free”) state and a buried, solvent-inaccessible (“bound”) state • Energetics of binding primaril ...
... • Typically, ca. 500 - 1500 Å2 of surface buried upon complex formation by two globular proteins • Epitopes on protein surface thus may have a “hybrid” character, compatible with both a solvent-exposed (‘free”) state and a buried, solvent-inaccessible (“bound”) state • Energetics of binding primaril ...
Document
... unfolded protein in the interface. However, as far as we know, one cannot actually achieve this state with constitutive membrane proteins because of the solubility problems nor with small non-constitutive membrane-active peptides because binding usually induces secondary structure (partitioning-fold ...
... unfolded protein in the interface. However, as far as we know, one cannot actually achieve this state with constitutive membrane proteins because of the solubility problems nor with small non-constitutive membrane-active peptides because binding usually induces secondary structure (partitioning-fold ...
Biology Unit 1 Review Guide ANSWERS
... OTHERS – Evolution (change over time) and Internal organization (organ systems) ...
... OTHERS – Evolution (change over time) and Internal organization (organ systems) ...
Modelling protein Modelling protein--surface interactions: a surface interactions: a challenge for computations
... GolP force field in action • Studying liquid water on Au(111) • Simulating b-sheets proteins/peptides adhesion on Au(111) (in collaboration with S.Monti, M. Hoefling, K.Gottschalk) • Interpreting electron transfer measurements for cytochrome C mutants on gold (see poster by M. Siwko) • Studying pot ...
... GolP force field in action • Studying liquid water on Au(111) • Simulating b-sheets proteins/peptides adhesion on Au(111) (in collaboration with S.Monti, M. Hoefling, K.Gottschalk) • Interpreting electron transfer measurements for cytochrome C mutants on gold (see poster by M. Siwko) • Studying pot ...
Medical Physics and Statistics
... The first prize of the Idaho Falls High School Science Fair was awarded on April 26 to a student of Eagle Rock High School. The student wanted to demonstrate the extent to which the public is manipulated by vague references to science in generating environmental concern. He prepared a proposal for ba ...
... The first prize of the Idaho Falls High School Science Fair was awarded on April 26 to a student of Eagle Rock High School. The student wanted to demonstrate the extent to which the public is manipulated by vague references to science in generating environmental concern. He prepared a proposal for ba ...
Problem Set 1
... α-helix, compared to that of one at the COOH-terminus, is likely to be: a) increased b) decreased c) similar to each other, but not to the free amino acid d) similar to the free amino acid ...
... α-helix, compared to that of one at the COOH-terminus, is likely to be: a) increased b) decreased c) similar to each other, but not to the free amino acid d) similar to the free amino acid ...
L2_Principle of protein folding in the cellular environment
... Two rings of 7x2GroEL proteins (shown in blue and green) with a cap (just on one side) of GroES proteins (red and yellow) ...
... Two rings of 7x2GroEL proteins (shown in blue and green) with a cap (just on one side) of GroES proteins (red and yellow) ...
Passive and active transport
... a pump specific for certain amino acids but can not transport glucose. Others can pump glucose but not ...
... a pump specific for certain amino acids but can not transport glucose. Others can pump glucose but not ...
Protein Folding Questions only
... - Basic sidechains contain __________________ atoms. This is called an __________________ functional group. - Hydrophilic sidechains have various combinations of ____________. An exception to this observation is: ...
... - Basic sidechains contain __________________ atoms. This is called an __________________ functional group. - Hydrophilic sidechains have various combinations of ____________. An exception to this observation is: ...
A type of passive transport that requires a transport protein
... where there is no EQUILIBRIUM another substance, net change in such as sugar or movement. This is ...
... where there is no EQUILIBRIUM another substance, net change in such as sugar or movement. This is ...
Solutions
... Examples for emulsoids include protein, starch and egg white a layer of solvent solutions. 2- Suspensoids They are lyophobic (solvent hating) colloids. If the solvent is water they are called hydrophobic colloids. They are less viscid than emulsoids. They are less stable and easily precipitated as t ...
... Examples for emulsoids include protein, starch and egg white a layer of solvent solutions. 2- Suspensoids They are lyophobic (solvent hating) colloids. If the solvent is water they are called hydrophobic colloids. They are less viscid than emulsoids. They are less stable and easily precipitated as t ...
Biochemistry
... then protein complexes, protein structural motifs to understand protein functions, nucleotides to DNA and its replication; carbohydrates to polysaccharides, etc. ...
... then protein complexes, protein structural motifs to understand protein functions, nucleotides to DNA and its replication; carbohydrates to polysaccharides, etc. ...
Molecules HW Lesson 7BYBHW
... What is the relationship between atoms and molecules? What are the biologically relevant molecules? What types of molecules to drugs bind to? How do related molecules compare to each other, how do they differ? How does one read and interpret a molecular diagram? ...
... What is the relationship between atoms and molecules? What are the biologically relevant molecules? What types of molecules to drugs bind to? How do related molecules compare to each other, how do they differ? How does one read and interpret a molecular diagram? ...
C.I.R.L Regulation of Body Temperature And Energy Production In
... » Fats serve as the body’s energy reserve. They are insoluble in water (nonpolar) which prevent them from being flushed out in urine, and they are very energy rich. ...
... » Fats serve as the body’s energy reserve. They are insoluble in water (nonpolar) which prevent them from being flushed out in urine, and they are very energy rich. ...
Protein Structure 2 - Interactions - Hydrolysis
... Fibrous proteins – long, stretched out (insoluble in water). Mostly structural. (3 α-helixes coiled together.) Ex. α-Keratins in hair, wool, skin and nails. ...
... Fibrous proteins – long, stretched out (insoluble in water). Mostly structural. (3 α-helixes coiled together.) Ex. α-Keratins in hair, wool, skin and nails. ...
CHM_101_TUTORIAL_QUESTIONS_1
... 5. Screening & Shielding effect: Presence of other orbits between nucleus and last orbit decreases the nuclear attraction. This effect is called screening effect but electron-electron repulsion is called shielding effect which also decreases the nuclear attraction. Due to presence of these effects i ...
... 5. Screening & Shielding effect: Presence of other orbits between nucleus and last orbit decreases the nuclear attraction. This effect is called screening effect but electron-electron repulsion is called shielding effect which also decreases the nuclear attraction. Due to presence of these effects i ...
Terminology 1
... The weight of one molecule of the substance relative to the weight of carbon atom taken as 12 The molecular weight is the sum of the atomic weights of the elements present in one molecule of the substance When molecular weight is expressed in grams, it is termed as gram molecule ...
... The weight of one molecule of the substance relative to the weight of carbon atom taken as 12 The molecular weight is the sum of the atomic weights of the elements present in one molecule of the substance When molecular weight is expressed in grams, it is termed as gram molecule ...
2nd Semester Exam Review
... little volume relative to the volume of empty space around them – Gas molecules are very far apart and therefore don’t experience attractive or repulsive forces. ...
... little volume relative to the volume of empty space around them – Gas molecules are very far apart and therefore don’t experience attractive or repulsive forces. ...
What is the average % of protein in Grade 1 oats
... What might you expect a horse to look like if he were deficient in protein? ...
... What might you expect a horse to look like if he were deficient in protein? ...
Thermochimica Acta Thermodynamics of hydrogen bonding and van
... by variation of anions and cations nature. However, general relationships between the solubility of different organic compounds and structure of ionic liquids should be known in order to undertake such procedure. Meanwhile, a substance’s solubility in a given solvent is determined by the intermolecu ...
... by variation of anions and cations nature. However, general relationships between the solubility of different organic compounds and structure of ionic liquids should be known in order to undertake such procedure. Meanwhile, a substance’s solubility in a given solvent is determined by the intermolecu ...
BIO1019 Lecture 20 - phospholipids
... • Driven by concentration gradient Active Transport • Depends of the presence of specific transport proteins • Transport requires energy • Transport can be against a concentration gradient ...
... • Driven by concentration gradient Active Transport • Depends of the presence of specific transport proteins • Transport requires energy • Transport can be against a concentration gradient ...
Proteins File
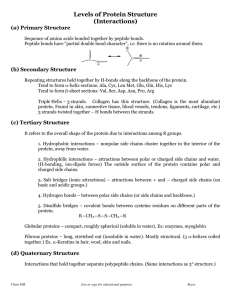
... Tertiary structure This is the overall arrangement of all the atoms in the protein, i.e., its overall shape. Every protein has a natural tertiary structure – most stable shape. It is active only in that shape. Tertiary structure is determined by primary structure. Tertiary structure is stabil ...
... Tertiary structure This is the overall arrangement of all the atoms in the protein, i.e., its overall shape. Every protein has a natural tertiary structure – most stable shape. It is active only in that shape. Tertiary structure is determined by primary structure. Tertiary structure is stabil ...
Chemistry 121 Mines PS13-1
... Reasoning/Comments: The solubility of gases depends on both temperature and pressure (unlike solids and liquids, which are only significantly dependent on temperature). It is most clear to see the effects of each if you start out with a system at dynamic equilibrium at a given T and P and then imagi ...
... Reasoning/Comments: The solubility of gases depends on both temperature and pressure (unlike solids and liquids, which are only significantly dependent on temperature). It is most clear to see the effects of each if you start out with a system at dynamic equilibrium at a given T and P and then imagi ...