Glycolysis in the Cytoplasm
... -splits one molecule of glucose (6 carbon molecule) into two molecules of a 3-carbon acid - pyruvic acid (pyruvate). Glycolysis occurs in two phases: 1. Glycolysis I - Energy Investment Phase ATP is used to split the 6-carbon molecule into two 3-carbon molecules 2. Glycolysis II - Energy Payoff Phas ...
... -splits one molecule of glucose (6 carbon molecule) into two molecules of a 3-carbon acid - pyruvic acid (pyruvate). Glycolysis occurs in two phases: 1. Glycolysis I - Energy Investment Phase ATP is used to split the 6-carbon molecule into two 3-carbon molecules 2. Glycolysis II - Energy Payoff Phas ...
Metabolism
... A substance is oxidized when it loses one or more electrons A substance is reduced when it gains one or more electrons Oxidation-reduction reactions are controlled by enzymes Antioxidants – compounds that donate electrons to oxidized compounds, putting them into a more reduced (stable) state ...
... A substance is oxidized when it loses one or more electrons A substance is reduced when it gains one or more electrons Oxidation-reduction reactions are controlled by enzymes Antioxidants – compounds that donate electrons to oxidized compounds, putting them into a more reduced (stable) state ...
Energy Metabolism - Georgia Institute of Technology
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
AP Bio A final exam study guide
... Explain the difference between polar and nonpolar molecules relating this property to interactions with water molecules (hydrophilic vs. hydrophobic). Give examples. ...
... Explain the difference between polar and nonpolar molecules relating this property to interactions with water molecules (hydrophilic vs. hydrophobic). Give examples. ...
Mitochondria
... bread is starch, a polysaccharide that is readily broken down by the digestive system into its component monosaccharide, glucose. The resulting glucose molecules can be oxidized to produce ATP (catabolism) or they can be bound together to make another polysaccharide, glycogen (anabolism). Glycogen i ...
... bread is starch, a polysaccharide that is readily broken down by the digestive system into its component monosaccharide, glucose. The resulting glucose molecules can be oxidized to produce ATP (catabolism) or they can be bound together to make another polysaccharide, glycogen (anabolism). Glycogen i ...
Cellular Respiration Chapter 9
... •Oxidation is the loss of electrons; electrons are removed from hydrogen atoms contained in glucose. •Reduction is the gain of electrons; oxygen atoms accept hydrogen and electrons forming water H2O. ...
... •Oxidation is the loss of electrons; electrons are removed from hydrogen atoms contained in glucose. •Reduction is the gain of electrons; oxygen atoms accept hydrogen and electrons forming water H2O. ...
Cellular Respiration Powerpoint1
... Requires an input of energy (ATP) Occurs in the cytoplasm Is the splitting of sugar (glucose) Releases only a small amount of energy but the process is fast; can produce thousands of ATP molecules in a few milliseconds Produces two molecules of a 3 carbon compound, PGA The net yield is 2 ATP, 2 NADH ...
... Requires an input of energy (ATP) Occurs in the cytoplasm Is the splitting of sugar (glucose) Releases only a small amount of energy but the process is fast; can produce thousands of ATP molecules in a few milliseconds Produces two molecules of a 3 carbon compound, PGA The net yield is 2 ATP, 2 NADH ...
No Slide Title
... passed along inner membrane • Energy used to pump H+ ions from matrix into space between inner & outer membrane • High concentration of H+ is maintained outside of inner membrane • ATP synthesis occurs as H+ diffuses through a special H+ channel in inner membrane ...
... passed along inner membrane • Energy used to pump H+ ions from matrix into space between inner & outer membrane • High concentration of H+ is maintained outside of inner membrane • ATP synthesis occurs as H+ diffuses through a special H+ channel in inner membrane ...
25-1
... passed along inner membrane • Energy used to pump H+ ions from matrix into space between inner & outer membrane • High concentration of H+ is maintained outside of inner membrane • ATP synthesis occurs as H+ diffuses through a special H+ channel in inner membrane ...
... passed along inner membrane • Energy used to pump H+ ions from matrix into space between inner & outer membrane • High concentration of H+ is maintained outside of inner membrane • ATP synthesis occurs as H+ diffuses through a special H+ channel in inner membrane ...
(1) Peter Mitchell and the Chemiosmotic Theory
... existence of a link between the Krebs cycle and the catabolism of fatty acids In he cell. • In 1949, Morris Friedkin, together with his PhD supervisor, Albert Lehninger , showed the existence of a connection between different metabolic pathways for coenzyme NADH to oxygen as a source of energy in ox ...
... existence of a link between the Krebs cycle and the catabolism of fatty acids In he cell. • In 1949, Morris Friedkin, together with his PhD supervisor, Albert Lehninger , showed the existence of a connection between different metabolic pathways for coenzyme NADH to oxygen as a source of energy in ox ...
A.) There are three different categories of cellular poisons that affect
... – During this stage, electrons are shuttled through the electron transport chain – As a result, ATP is generated through oxidative phosphorylation associated ...
... – During this stage, electrons are shuttled through the electron transport chain – As a result, ATP is generated through oxidative phosphorylation associated ...
Cell Respiration - Biology Junction
... 1) is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed from carrier to carrier until received by oxygen; 2) passes electrons from higher to lower energy states, allowing energy to be released and stored for ATP production; 8.2 Outside ...
... 1) is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed from carrier to carrier until received by oxygen; 2) passes electrons from higher to lower energy states, allowing energy to be released and stored for ATP production; 8.2 Outside ...
Comparing Fermentation with Anaerobic and
... operate In that case, glycolysis couples with fermentation or anaerobic respiration to produce ATP ...
... operate In that case, glycolysis couples with fermentation or anaerobic respiration to produce ATP ...
Chapter 9—Cellular Respiration: Harvesting Chemical Energy
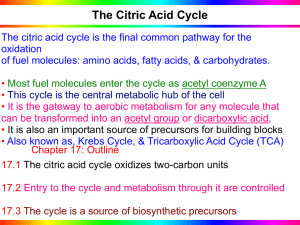
... Concept 9.3: The citric acid cycle completes the energy-yielding oxidation of organic molecules C. Citric Acid Cycle Most of the chemical energy originally stored in glucose still resides in the two pyruvate molecules produced by glycolysis. The fate of pyruvate depends upon the presence or absen ...
... Concept 9.3: The citric acid cycle completes the energy-yielding oxidation of organic molecules C. Citric Acid Cycle Most of the chemical energy originally stored in glucose still resides in the two pyruvate molecules produced by glycolysis. The fate of pyruvate depends upon the presence or absen ...
CELLULAR RESPIRATION
... chain so that their energy can be used to convert ADP into ATP These reactions require oxygen, which accepts the H+ ions to form water Occurs in the mitochondria The entire process of aerobic respiration produces 36 ATP molecules ...
... chain so that their energy can be used to convert ADP into ATP These reactions require oxygen, which accepts the H+ ions to form water Occurs in the mitochondria The entire process of aerobic respiration produces 36 ATP molecules ...
CELLULAR RESPIRATION
... chain so that their energy can be used to convert ADP into ATP These reactions require oxygen, which accepts the H+ ions to form water Occurs in the mitochondria The entire process of aerobic respiration produces 36 ATP molecules ...
... chain so that their energy can be used to convert ADP into ATP These reactions require oxygen, which accepts the H+ ions to form water Occurs in the mitochondria The entire process of aerobic respiration produces 36 ATP molecules ...
File
... electrons from carbon fuels 1. The cycle itself neither generates ATP nor includes O2 as a reactant 1. Instead, it removes electrons from acetyl CoA & uses them to form NADH & FADH2 (high-energy electron carriers) 1. In oxidative phosphorylation, electrons from reoxidation of NADH & FADH2 flow throu ...
... electrons from carbon fuels 1. The cycle itself neither generates ATP nor includes O2 as a reactant 1. Instead, it removes electrons from acetyl CoA & uses them to form NADH & FADH2 (high-energy electron carriers) 1. In oxidative phosphorylation, electrons from reoxidation of NADH & FADH2 flow throu ...
No Slide Title
... ATP, NADH, and two pyruvates are the end products of glycolysis. It’s vital to know the reactants and products for each process of cellular respiration and photosynthesis! ...
... ATP, NADH, and two pyruvates are the end products of glycolysis. It’s vital to know the reactants and products for each process of cellular respiration and photosynthesis! ...
CellularRespirationglycolysis
... uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane – These ions store potential energy ...
... uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane – These ions store potential energy ...
EXAM 2 Fall2007.doc
... e. entropy has been decreased. 7. The second law of thermodynamics states that for chemical reactions: a. entropy always increases. b. entropy always decreases. c. free energy always increases. d. free energy always decreases. e. anabolic reactions must always be paired with catabolic reactions. 8. ...
... e. entropy has been decreased. 7. The second law of thermodynamics states that for chemical reactions: a. entropy always increases. b. entropy always decreases. c. free energy always increases. d. free energy always decreases. e. anabolic reactions must always be paired with catabolic reactions. 8. ...
8.3 Cellular Respiration
... • NADH & FADH2 drop off electrons • Electrons move down the electron transport chain • pulled down the chain by oxygen • Hydrogen ions diffuse from an area of high concentration (outside the membrane) to an area of low concentration (inner-membrane space) through ATP synthase. • ATP synthase is like ...
... • NADH & FADH2 drop off electrons • Electrons move down the electron transport chain • pulled down the chain by oxygen • Hydrogen ions diffuse from an area of high concentration (outside the membrane) to an area of low concentration (inner-membrane space) through ATP synthase. • ATP synthase is like ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.