Atomic Structure - Hudson City School District
... • 1st shell – 1 pair • 2nd shell – e- space far apart, singles, then pair • 3rd shell – e- space far apart, singles, then pair ...
... • 1st shell – 1 pair • 2nd shell – e- space far apart, singles, then pair • 3rd shell – e- space far apart, singles, then pair ...
25 NUCLEAR CHEMISTRY NOTES __ /__ pts
... 5. Which two have the same mass? (alpha, protons, electrons, beta) 6. Which two have the same charge? (protons, electrons, neutrons, beta) 7. Which two have no charge? (protons, electrons, neutrons, gamma) 8. Which two have no mass? (protons, electrons, neutrons, gamma) 9. Which has the biggest mass ...
... 5. Which two have the same mass? (alpha, protons, electrons, beta) 6. Which two have the same charge? (protons, electrons, neutrons, beta) 7. Which two have no charge? (protons, electrons, neutrons, gamma) 8. Which two have no mass? (protons, electrons, neutrons, gamma) 9. Which has the biggest mass ...
Review Questions
... A. All scientists must agree with an idea for it to be considered a theory. B. Theories can be proven correct. C. Scientists usually create an entirely new theory when evidence is found that ...
... A. All scientists must agree with an idea for it to be considered a theory. B. Theories can be proven correct. C. Scientists usually create an entirely new theory when evidence is found that ...
Distinguishing Among Atoms Worksheet
... atomic mass units. ___________________ 14. Circle the letter of each statement that is true about the average atomic mass of an element and the relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific ...
... atomic mass units. ___________________ 14. Circle the letter of each statement that is true about the average atomic mass of an element and the relative abundance of its isotopes. a. In nature, most elements occur as a mixture of two or more isotopes. b. Isotopes of an element do not have a specific ...
Chapter 2 – Atoms and Elements
... In 1794, Joseph Proust (1754-1826) demonstrated the law of definite proportions (aka law of constant composition): In a given chemical compound, the proportions by mass of the elements that compose it are fixed, independent of the origin of the compound or its mode of preparation. In 1808, John Dalt ...
... In 1794, Joseph Proust (1754-1826) demonstrated the law of definite proportions (aka law of constant composition): In a given chemical compound, the proportions by mass of the elements that compose it are fixed, independent of the origin of the compound or its mode of preparation. In 1808, John Dalt ...
Bean Bag Lab
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
... Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different masses. These atoms were call iso ...
Atom? - Its All about the Science
... propose about the atom? • All substances are made up of atoms which are small particles that cannot be created, divided, or destroyed. • Atoms of the same element are exactly alike and atoms of different elements are different. • Atoms join with other atoms to form different substances ...
... propose about the atom? • All substances are made up of atoms which are small particles that cannot be created, divided, or destroyed. • Atoms of the same element are exactly alike and atoms of different elements are different. • Atoms join with other atoms to form different substances ...
Chemistry 2810 Answers to the Second assignment Topic: Atomic
... All the magnitudes increase from left to right across the period, since each trend reflects the increasing Z* as electrons are being added to orbitals of the same shell. For IP, this reflects the difficulty of removing an electron. For EG enthalpy, this reflects energy released when an additional el ...
... All the magnitudes increase from left to right across the period, since each trend reflects the increasing Z* as electrons are being added to orbitals of the same shell. For IP, this reflects the difficulty of removing an electron. For EG enthalpy, this reflects energy released when an additional el ...
Atomic Theory Notes
... Atoms combine with each other in specific ratios to make compounds Chemical reactions occur when atoms are separated, joined, or rearranged, but the atoms of one element are not changed into the atoms of another by chemical reaction ...
... Atoms combine with each other in specific ratios to make compounds Chemical reactions occur when atoms are separated, joined, or rearranged, but the atoms of one element are not changed into the atoms of another by chemical reaction ...
04 Mass Spectrometer and Isotopes
... been different if your buggy was not quite so full? How easily you can change the direction of your movement is affected by how much mass you have. Isotopes have different masses! Maybe we can use this to determine the mass of different isotopes! ...
... been different if your buggy was not quite so full? How easily you can change the direction of your movement is affected by how much mass you have. Isotopes have different masses! Maybe we can use this to determine the mass of different isotopes! ...
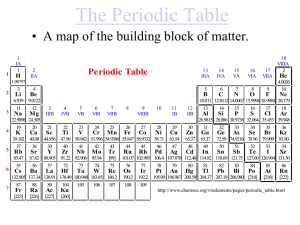
The Periodic Table - River Dell Regional School District
... • 3. Valence electrons and group number – a. Representative element’s group number and number of valence electrons it contains also are related. – Ie: Na has one e- on valence shell – Lets work on some ...
... • 3. Valence electrons and group number – a. Representative element’s group number and number of valence electrons it contains also are related. – Ie: Na has one e- on valence shell – Lets work on some ...
7.1 The Periodic Table
... • Characteristics that you can see through direct observation are called physical properties. • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physical properties. ...
... • Characteristics that you can see through direct observation are called physical properties. • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physical properties. ...
Parts of the Atom & History of Discovery PPT
... The atoms of a given element are different from those of any other element. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms. Atoms are indivisible in chemical processes. That is ,atoms are not c ...
... The atoms of a given element are different from those of any other element. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms. Atoms are indivisible in chemical processes. That is ,atoms are not c ...
The Structure of the Atom- Chapter 4, 3
... 1910 Rutherford discovers the nucleus through the gold foil experiment and that atoms are mostly _____________________ . Rutherford’s Atomic Model (sphere with dense middle center called the _________________ with electrons dispersed around it. ...
... 1910 Rutherford discovers the nucleus through the gold foil experiment and that atoms are mostly _____________________ . Rutherford’s Atomic Model (sphere with dense middle center called the _________________ with electrons dispersed around it. ...
Review 2 (Chapters 3,5, 10,11)
... Isotopes: When the Number of Neutrons Varies in an element A. Isotopes have the same chemical properties, but different masses B. Some isotopes are more prevalent than others C. Chemical symbol with mass number indicates which isotope D. Some elements have many isotopes, some very few Elements: Defi ...
... Isotopes: When the Number of Neutrons Varies in an element A. Isotopes have the same chemical properties, but different masses B. Some isotopes are more prevalent than others C. Chemical symbol with mass number indicates which isotope D. Some elements have many isotopes, some very few Elements: Defi ...
Chapter Twelve: Atoms and the Periodic Table
... • Mendeleev arranged the elements in order of increasing mass so that elements with similar properties were in the same column. • Mendeleev used the properties of existing elements to predict properties of undiscovered elements. • The close match between Mendeleev’s predictions and the actual prope ...
... • Mendeleev arranged the elements in order of increasing mass so that elements with similar properties were in the same column. • Mendeleev used the properties of existing elements to predict properties of undiscovered elements. • The close match between Mendeleev’s predictions and the actual prope ...
2 - grade11chemistry
... 1. Determine the number of valence electrons of the element. Recall: The number of valence electrons (outermost electrons) of an element is equal to its group number. 2. Draw the symbol of the element. The symbol of the element is used to represent the core (protons and neutrons) and the inner elect ...
... 1. Determine the number of valence electrons of the element. Recall: The number of valence electrons (outermost electrons) of an element is equal to its group number. 2. Draw the symbol of the element. The symbol of the element is used to represent the core (protons and neutrons) and the inner elect ...
The Atom
... Matter (in our world) is composed of combinations of about 100 basic substances called elements 109 elements have been discovered and isolated 88 are found in nature 21 are (synthetic) man-made Oxygen most abundant element (by mass) on earth ...
... Matter (in our world) is composed of combinations of about 100 basic substances called elements 109 elements have been discovered and isolated 88 are found in nature 21 are (synthetic) man-made Oxygen most abundant element (by mass) on earth ...
Periodic_Tendancies
... electrons • Most electronegative atoms are in upper right corner of periodic table (fluorine) • That’s why atoms on the right gain electrons; they pull electrons from the metals on the left. ...
... electrons • Most electronegative atoms are in upper right corner of periodic table (fluorine) • That’s why atoms on the right gain electrons; they pull electrons from the metals on the left. ...
atom - BobcatChemistry
... The ancient Greeks tried to explain matter, but the scientific study of the atom began with John Dalton in the early 1800's. An atom is made of a nucleus containing protons and neutrons; electrons move around the nucleus. The number of protons and the mass number define the type of atom. Unstable at ...
... The ancient Greeks tried to explain matter, but the scientific study of the atom began with John Dalton in the early 1800's. An atom is made of a nucleus containing protons and neutrons; electrons move around the nucleus. The number of protons and the mass number define the type of atom. Unstable at ...
Chapter 2 – Atoms and Elements
... • Groups 4-11 are the ‘true’ transition metals in that they lose electrons to form coloured compounds in which the metal atom has a positive charge • Groups 11 and 12 mimic the behaviour of Groups 1 and 2 respectively but are less reactive • the metals in the middle of the transition groups are hard ...
... • Groups 4-11 are the ‘true’ transition metals in that they lose electrons to form coloured compounds in which the metal atom has a positive charge • Groups 11 and 12 mimic the behaviour of Groups 1 and 2 respectively but are less reactive • the metals in the middle of the transition groups are hard ...
Name______________________________ (First and Last
... pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard of nucleons and quarks. Nuclear chemist ...
... pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard of nucleons and quarks. Nuclear chemist ...
Getting to Know: Atomic Structure and Elements
... What are some other properties of elements and atoms? An atom cannot be divided further and still be identified as an atom of a particular element. For example, if a silver atom were split into protons, neutrons, and electrons, it would no longer be silver. It would just be a collection of subatomic ...
... What are some other properties of elements and atoms? An atom cannot be divided further and still be identified as an atom of a particular element. For example, if a silver atom were split into protons, neutrons, and electrons, it would no longer be silver. It would just be a collection of subatomic ...
The Atom Notes
... of electrons will be the same as protons. ION = a charged atom (unequal number of protons and electrons) +1 charge = the atom LOST one electron -1 charge = the atom GAINED one electron +2 charge = LOST 2 electrons -2 charge = GAINED 2 electrons and so on ...
... of electrons will be the same as protons. ION = a charged atom (unequal number of protons and electrons) +1 charge = the atom LOST one electron -1 charge = the atom GAINED one electron +2 charge = LOST 2 electrons -2 charge = GAINED 2 electrons and so on ...
chapter 2 - Scranton Prep Biology
... ' Electron conJiguration= Distributionof electronsin an atom's electron shells The first l8 elementsof a periodicchartare arrangedsequentially by atomic numberinto threerows (periods).In referenceto theserepresentotive elements, note the following: ' Outermostshell of theseatomsnever have more than ...
... ' Electron conJiguration= Distributionof electronsin an atom's electron shells The first l8 elementsof a periodicchartare arrangedsequentially by atomic numberinto threerows (periods).In referenceto theserepresentotive elements, note the following: ' Outermostshell of theseatomsnever have more than ...