Periodic Law
... The groups in the periodic table have "A" and "B" designations. The elements in the A groups, which appear in two parts—two at the beginning and six at the end of the table—are known as the main group elements. Those in the B groups, which are in between the two A group divisions, are called transit ...
... The groups in the periodic table have "A" and "B" designations. The elements in the A groups, which appear in two parts—two at the beginning and six at the end of the table—are known as the main group elements. Those in the B groups, which are in between the two A group divisions, are called transit ...
Name Parts of an Atom Worksheet Date_______ Substances that
... Electrons are negatively charged and are located in rings or orbits spinning around the nucleus. The number of protons and electrons is always equal. This equality is important so that the atom is neither positively nor negatively charged. It is said to be neutral. The third part of the atom is the ...
... Electrons are negatively charged and are located in rings or orbits spinning around the nucleus. The number of protons and electrons is always equal. This equality is important so that the atom is neither positively nor negatively charged. It is said to be neutral. The third part of the atom is the ...
Atomic Theory - Valhalla High School
... All electron configurations on the Periodic Table are NUETRAL ( p = e) ...
... All electron configurations on the Periodic Table are NUETRAL ( p = e) ...
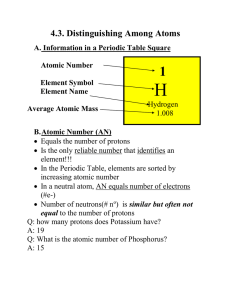
Notes 4.3 filled in
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
Chapter 5 Atomic Structure and the Periodic Table
... amu (atomic mass unit = 1.66 x 10-24 grams) The electron was discovered by the English physicist Sir Joseph J. Thomson around 1897 with the use of a cathode ray tube. A cathode ray tube is similar to your TV. It has an anode (negative electrode) and a cathode (positive electrode). These are enclosed ...
... amu (atomic mass unit = 1.66 x 10-24 grams) The electron was discovered by the English physicist Sir Joseph J. Thomson around 1897 with the use of a cathode ray tube. A cathode ray tube is similar to your TV. It has an anode (negative electrode) and a cathode (positive electrode). These are enclosed ...
Atoms of different elements are
... Understand the inverse relationship between wavelength and frequency, and the direct relationship between energy and frequency Analyze diagrams related to the Bohr model of the hydrogen atom in terms of allowed, discrete energy levels in the emission spectrum Describe the electron cloud of the atom ...
... Understand the inverse relationship between wavelength and frequency, and the direct relationship between energy and frequency Analyze diagrams related to the Bohr model of the hydrogen atom in terms of allowed, discrete energy levels in the emission spectrum Describe the electron cloud of the atom ...
Chemistry: Matter and Change
... beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus determines its stability. ...
... beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus determines its stability. ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
Ch. 3 - My CCSD
... observed X-rays were organized in order of increasing frequency suggested to Moseley a regular increase in the positive charge on the nuclei of the atoms. He called this positive nuclear chargethe Atomic Number of the element ...
... observed X-rays were organized in order of increasing frequency suggested to Moseley a regular increase in the positive charge on the nuclei of the atoms. He called this positive nuclear chargethe Atomic Number of the element ...
Chemistry: Matter and Change
... beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus determines its stability. ...
... beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus determines its stability. ...
atomic number
... observed X-rays were organized in order of increasing frequency suggested to Moseley a regular increase in the positive charge on the nuclei of the atoms. He called this positive nuclear chargethe Atomic Number of the element ...
... observed X-rays were organized in order of increasing frequency suggested to Moseley a regular increase in the positive charge on the nuclei of the atoms. He called this positive nuclear chargethe Atomic Number of the element ...
Electron Proton Neutron
... The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11. ...
... The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11. ...
The Periodic Table - Duplin County Schools
... Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used every where in the world Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element. ...
... Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used every where in the world Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element. ...
View PDF
... and an atom in an excited state? a. The atom in the ground state has less energy and is less stable than the atom in an excited state. b. The atom in an excited state has one fewer electron than the atom in the ground state. c. The atom in an excited state has more energy and is less stable than the ...
... and an atom in an excited state? a. The atom in the ground state has less energy and is less stable than the atom in an excited state. b. The atom in an excited state has one fewer electron than the atom in the ground state. c. The atom in an excited state has more energy and is less stable than the ...
Chapters 1-4 Numbers and Measurements in Chemistry Units SI
... • Because carbon compounds can become quite large, organic compounds are described simply and unambiguously using line structures, where carbons and hydrogens are not explicitly shown. – Each corner or end of a line is a carbon. – Hydrogen y g atoms on carbon atoms are implied. p Carbon makes four b ...
... • Because carbon compounds can become quite large, organic compounds are described simply and unambiguously using line structures, where carbons and hydrogens are not explicitly shown. – Each corner or end of a line is a carbon. – Hydrogen y g atoms on carbon atoms are implied. p Carbon makes four b ...
Atom
... • Each element has a unique name and symbol. • All of this data, and more, are collected in an organized table called the periodic table of elements. ...
... • Each element has a unique name and symbol. • All of this data, and more, are collected in an organized table called the periodic table of elements. ...
Ch. 10: States of Matter Solids
... atomic theory of the time, the alpha particles should pass through the foil with only a slight deflection ...
... atomic theory of the time, the alpha particles should pass through the foil with only a slight deflection ...
The Fundamental Ideas in Chemistry
... Recall that chemical reactions can be represented by word equations or symbol equations. Describe how no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants. Calculate the mass of a reactant or product from information about the masses of th ...
... Recall that chemical reactions can be represented by word equations or symbol equations. Describe how no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants. Calculate the mass of a reactant or product from information about the masses of th ...
specific vocabulary of the unit
... includes helium, neon, argon, krypton, xenon, radon, and element 118. These elements are referred to as "inert" or "noble" because they do not easily form compounds with other elements. Transition elements /træn'zɪʃən//'eləmənts / A class of elements occurring in the periodic table in three series: ...
... includes helium, neon, argon, krypton, xenon, radon, and element 118. These elements are referred to as "inert" or "noble" because they do not easily form compounds with other elements. Transition elements /træn'zɪʃən//'eləmənts / A class of elements occurring in the periodic table in three series: ...
Atomic Structure The Nucleus The Electrons Atomic Theory
... they have different atoms. Pure gold mixed with pure copper forms rose gold. The gold and copper atoms combine in a simple numerical ratio. Dalton's theory has not proven to be correct under all circumstances. The first rule was proven incorrect when scientists divided atoms in a process called nucl ...
... they have different atoms. Pure gold mixed with pure copper forms rose gold. The gold and copper atoms combine in a simple numerical ratio. Dalton's theory has not proven to be correct under all circumstances. The first rule was proven incorrect when scientists divided atoms in a process called nucl ...
Atomic Structure & The Periodic Table
... In the early 1800’s British scientist John Dalton proposed that each element is made up of tiny particles called atoms. Dalton stated that all of the atoms of a particular element are identical but are different from atoms of all other elements. Daltons theory also assumed that atoms could not be di ...
... In the early 1800’s British scientist John Dalton proposed that each element is made up of tiny particles called atoms. Dalton stated that all of the atoms of a particular element are identical but are different from atoms of all other elements. Daltons theory also assumed that atoms could not be di ...
Atomic Structure-PRACTICE TEST
... TRUE or FALSE - the atomic mass increases by ONE from element to element (atomic number) TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRU ...
... TRUE or FALSE - the atomic mass increases by ONE from element to element (atomic number) TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRU ...
Atomic Structure Unit Test 2016
... b. two, if they have the same spin c. one d. no more than eight ____ 54. How many more electrons are needed to completely fill the third main energy level if it already contains 8 electrons? a. 0 c. 10 b. 8 d. 22 ____ 55. The atomic sublevel with the next highest energy after 4p is a. 4d. c. 5p. b. ...
... b. two, if they have the same spin c. one d. no more than eight ____ 54. How many more electrons are needed to completely fill the third main energy level if it already contains 8 electrons? a. 0 c. 10 b. 8 d. 22 ____ 55. The atomic sublevel with the next highest energy after 4p is a. 4d. c. 5p. b. ...
Chemistry A - Montgomery County Public Schools
... describe the characteristics of protons, neutrons and electrons in terms of location, charge and mass. illustrate the structure of the atom by using the Bohr model, including the charge, relative mass and location of the sub-atomic particles. use atomic mass, atomic number, and charge to ident ...
... describe the characteristics of protons, neutrons and electrons in terms of location, charge and mass. illustrate the structure of the atom by using the Bohr model, including the charge, relative mass and location of the sub-atomic particles. use atomic mass, atomic number, and charge to ident ...