1) Molecular Compounds
... Mass. He observed that the different groups of elements had similar properties, and that these properties periodically repeated when arranged by atomic mass. So he arranged the elements in a table by their increasing mass in horizontal rows, and put elements with similar properties in vertical colum ...
... Mass. He observed that the different groups of elements had similar properties, and that these properties periodically repeated when arranged by atomic mass. So he arranged the elements in a table by their increasing mass in horizontal rows, and put elements with similar properties in vertical colum ...
GED Chemistry Note 1[Atoms, Molecules and their properties]
... GED Chemistry Note 1[Atoms, Molecules and their properties] What is Atom? An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are very small; typical sizes are a ...
... GED Chemistry Note 1[Atoms, Molecules and their properties] What is Atom? An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are very small; typical sizes are a ...
Atomic Structure Test – Study Guide
... What is the electrical charge and position in the atom for each of the subatomic particles? 1. Electron - negative charge; located in a “cloud” rotating around the nucleus 2. Proton – positive charge; located in the center or nucleus of the atom 3. Neutron - no charge; located in the center or nucle ...
... What is the electrical charge and position in the atom for each of the subatomic particles? 1. Electron - negative charge; located in a “cloud” rotating around the nucleus 2. Proton – positive charge; located in the center or nucleus of the atom 3. Neutron - no charge; located in the center or nucle ...
11129_evl_ch1_ste_corr
... electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
... electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
Unit 2 - therrien
... When I have completed this section I will be able to: Distinguish between observation and theory and provide examples of how models are used to explain observations Demonstrate understanding of the origins of the periodic table and relate physical and chemical properties of the elements to the ...
... When I have completed this section I will be able to: Distinguish between observation and theory and provide examples of how models are used to explain observations Demonstrate understanding of the origins of the periodic table and relate physical and chemical properties of the elements to the ...
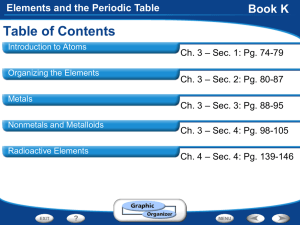
Elements and the Periodic Table
... • They are mixed with more common metals to produce alloys, which are a mixture of metal with one other element, usually another metal. ...
... • They are mixed with more common metals to produce alloys, which are a mixture of metal with one other element, usually another metal. ...
File
... Definition: Atomic Number: # of p+ in the nucleus Of an element is the _______________________________________________ of its atoms. No two elements have the _______ Same atomic number. This number defines an ________________. ...
... Definition: Atomic Number: # of p+ in the nucleus Of an element is the _______________________________________________ of its atoms. No two elements have the _______ Same atomic number. This number defines an ________________. ...
Democritus - davis.k12.ut.us
... • Considered the father of atomic theory for his idea that the world was made up of small, indivisible parts called “atomos”. • “Atomos” is the Greek word for “uncuttable.” This is where we get the current word “atom.” • His ideas were not popular, as most thinkers of the time held that the world wa ...
... • Considered the father of atomic theory for his idea that the world was made up of small, indivisible parts called “atomos”. • “Atomos” is the Greek word for “uncuttable.” This is where we get the current word “atom.” • His ideas were not popular, as most thinkers of the time held that the world wa ...
atoms - KMKunz
... • All atoms of a given element are alike in mass and other properties, but atoms of one element differ from the atoms of every other element • Compounds are formed when atoms of different elements unite in fixed proportions • A chemical reaction involves a rearrangement of atoms. No atoms are create ...
... • All atoms of a given element are alike in mass and other properties, but atoms of one element differ from the atoms of every other element • Compounds are formed when atoms of different elements unite in fixed proportions • A chemical reaction involves a rearrangement of atoms. No atoms are create ...
Atomic Structure Notes Packet
... John Dalton (1766-1844): used Democritus’s ideas and proposed the first atomic theory in 1803 based on experimentation, which included the following ideas: All ____________ consist of tiny indivisible particles called atoms. Atoms of the same element are ____________, different from those of any oth ...
... John Dalton (1766-1844): used Democritus’s ideas and proposed the first atomic theory in 1803 based on experimentation, which included the following ideas: All ____________ consist of tiny indivisible particles called atoms. Atoms of the same element are ____________, different from those of any oth ...
rev8thgrade - PAMS
... Atomic Structure: Isotopeshave the number of protons but different number of neutrons How many neutrons in the following isotopes? Hydrogen – 1 Helium – 4 Lithium – 6 Sodium – 22 ...
... Atomic Structure: Isotopeshave the number of protons but different number of neutrons How many neutrons in the following isotopes? Hydrogen – 1 Helium – 4 Lithium – 6 Sodium – 22 ...
Chemical Periodicity
... Second Ionization Energy energy required to remove the second outermost electron ** the higher the ionization energy, the harder it is to remove an electron ...
... Second Ionization Energy energy required to remove the second outermost electron ** the higher the ionization energy, the harder it is to remove an electron ...
Lesson 1: Alchemy and Atomic Models
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in simple whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in simple whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
Getting to Know: Atomic Structure and Elements
... elements. For example, bronze is a metal made of the elements copper and tin. Because it contains two types of atoms, bronze is not an element. ...
... elements. For example, bronze is a metal made of the elements copper and tin. Because it contains two types of atoms, bronze is not an element. ...
11 atomic number
... - Contains Protons and Neutrons ** Fact: If an atoms nucleus were the size of a pea, it would weigh 250 million tons. ** Protons-Located inside the nucleus of an atom -Has the charge of 1 fundamental unit (+1) -Give an element its atomic ______ -Have a weight of 1.6726 × 10−27 kg -Discovered in 1919 ...
... - Contains Protons and Neutrons ** Fact: If an atoms nucleus were the size of a pea, it would weigh 250 million tons. ** Protons-Located inside the nucleus of an atom -Has the charge of 1 fundamental unit (+1) -Give an element its atomic ______ -Have a weight of 1.6726 × 10−27 kg -Discovered in 1919 ...
1st mid unit test formative (pre-test)
... Metals are separated from non-metals by a staircase of elements called Metallica. Atomic number is the number of protons in an atom of an element. Atomic mass is the number of protons in an atom of an element. An ion is an atom or group of atoms with a negative charge or a positive charge. Noble gas ...
... Metals are separated from non-metals by a staircase of elements called Metallica. Atomic number is the number of protons in an atom of an element. Atomic mass is the number of protons in an atom of an element. An ion is an atom or group of atoms with a negative charge or a positive charge. Noble gas ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.

![Do Now - March [4-2], 2009 - stroh](http://s1.studyres.com/store/data/008519532_1-cab23fd6aae248311f653b62e7fe2161-300x300.png)
![GED Chemistry Note 1[Atoms, Molecules and their properties]](http://s1.studyres.com/store/data/015791288_1-b34903533007e866662649e94180f015-300x300.png)