Supplement on Lagrangian, Hamiltonian Mechanics
... that it says things in a very compact way. Note in particular that if we choose a path on which V does not change, the dV term in (11) is equal to zero, and dividing both sides by dS we arrive at the first of the two relations in (13); likewise by setting dS equal to zero we arrive at the second ter ...
... that it says things in a very compact way. Note in particular that if we choose a path on which V does not change, the dV term in (11) is equal to zero, and dividing both sides by dS we arrive at the first of the two relations in (13); likewise by setting dS equal to zero we arrive at the second ter ...
Review E: Simple Harmonic Motion and Mechanical Energy
... Proof: To verify the guess, take the first and second derivatives of the guess and substitute the second derivative into the SHO equation, dx dt = −ω A sin ( ωt ) + ω B cos ( ωt ) d 2 x dt 2 = −ω 2 A cos ( ωt ) − ω 2 B sin ( ωt ) = −ω 2 ( A cos ( ωt ) + B sin ( ωt ) ) = −ω 2 x (t ) ...
... Proof: To verify the guess, take the first and second derivatives of the guess and substitute the second derivative into the SHO equation, dx dt = −ω A sin ( ωt ) + ω B cos ( ωt ) d 2 x dt 2 = −ω 2 A cos ( ωt ) − ω 2 B sin ( ωt ) = −ω 2 ( A cos ( ωt ) + B sin ( ωt ) ) = −ω 2 x (t ) ...
chapter13
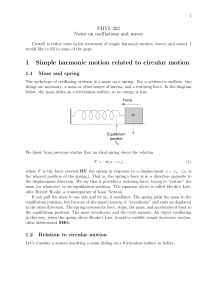
... Motion of the Spring-Mass System • Assume the object is initially pulled to a distance A and released from rest • As the object moves toward the equilibrium position, F and a decrease, but v increases • At x = 0, F and a are zero, but v is a maximum • The object’s momentum causes it to overshoot th ...
... Motion of the Spring-Mass System • Assume the object is initially pulled to a distance A and released from rest • As the object moves toward the equilibrium position, F and a decrease, but v increases • At x = 0, F and a are zero, but v is a maximum • The object’s momentum causes it to overshoot th ...
One Hundred Years of Quantum Physics
... the momentum. Because the slope varies from place to place, momentum is also “spread out.” The need to abandon a classical picture in which position and velocity can be determined with arbitrary accuracy in favor of a blurred picture of probabilities is at the heart of quantum mechanics. Measurement ...
... the momentum. Because the slope varies from place to place, momentum is also “spread out.” The need to abandon a classical picture in which position and velocity can be determined with arbitrary accuracy in favor of a blurred picture of probabilities is at the heart of quantum mechanics. Measurement ...
Unit 5 TEST REVIEW ALGEBRA 1a
... 1. The charge for a Hawkins and Young Photography Session includes an expense/travel fee of $185.00. The photographer then is paid an hourly rate of $250.00. Determine an equation that represents the total price of a session using x and y. What does the x represent? What does the y represent? equati ...
... 1. The charge for a Hawkins and Young Photography Session includes an expense/travel fee of $185.00. The photographer then is paid an hourly rate of $250.00. Determine an equation that represents the total price of a session using x and y. What does the x represent? What does the y represent? equati ...
Wave packet
.gif?width=300)
In physics, a wave packet (or wave train) is a short ""burst"" or ""envelope"" of localized wave action that travels as a unit. A wave packet can be analyzed into, or can be synthesized from, an infinite set of component sinusoidal waves of different wavenumbers, with phases and amplitudes such that they interfere constructively only over a small region of space, and destructively elsewhere. Each component wave function, and hence the wave packet, are solutions of a wave equation. Depending on the wave equation, the wave packet's profile may remain constant (no dispersion, see figure) or it may change (dispersion) while propagating.Quantum mechanics ascribes a special significance to the wave packet; it is interpreted as a probability amplitude, its norm squared describing the probability density that a particle or particles in a particular state will be measured to have a given position or momentum. The wave equation is in this case the Schrödinger equation. It is possible to deduce the time evolution of a quantum mechanical system, similar to the process of the Hamiltonian formalism in classical mechanics. The dispersive character of solutions of the Schrödinger equation has played an important role in rejecting Schrödinger's original interpretation, and accepting the Born rule.In the coordinate representation of the wave (such as the Cartesian coordinate system), the position of the physical object's localized probability is specified by the position of the packet solution. Moreover, the narrower the spatial wave packet, and therefore the better localized the position of the wave packet, the larger the spread in the momentum of the wave. This trade-off between spread in position and spread in momentum is a characteristic feature of the Heisenberg uncertainty principle,and will be illustrated below.