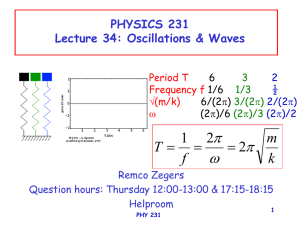
Surface Waves
... “there is no neat definition which would encompass all forms of wave which could glide or be guided along an interface”. However, the definitions which have been settled upon for this paper are as follows: Surface Wave Region: The region of interest in which the surface wave propagates. Surface Wave ...
... “there is no neat definition which would encompass all forms of wave which could glide or be guided along an interface”. However, the definitions which have been settled upon for this paper are as follows: Surface Wave Region: The region of interest in which the surface wave propagates. Surface Wave ...
Vacation-Assignment-Science-XII-2073
... 31. State and Explain Heisenberg’s uncertainty principle in terms of position and linear momentum of a particle. Use this principle to show that the electron is not constituent of the nucleus. 32. State and Explain Heisenberg’s uncertainty principle. Use this principle to show that the proton exists ...
... 31. State and Explain Heisenberg’s uncertainty principle in terms of position and linear momentum of a particle. Use this principle to show that the electron is not constituent of the nucleus. 32. State and Explain Heisenberg’s uncertainty principle. Use this principle to show that the proton exists ...
Quantum Mechanics in One Dimension
... This function ⌿(x, 0), called a Gaussian function, has a single maximum at x ⫽ 0 and decays smoothly to zero on either side of this point (Fig. 6.4a). The width of this Gaussian packet becomes larger with increasing ␣. Accordingly, it is reasonable to identify ␣ with ⌬x, the initial degree of locali ...
... This function ⌿(x, 0), called a Gaussian function, has a single maximum at x ⫽ 0 and decays smoothly to zero on either side of this point (Fig. 6.4a). The width of this Gaussian packet becomes larger with increasing ␣. Accordingly, it is reasonable to identify ␣ with ⌬x, the initial degree of locali ...
Sample pages 1 PDF
... exactly two independent solutions, say x1 (t) and x2 (t), meaning one is not a constant multiple of the other; they are not proportional. Such a set of solutions, x1 (t), x2 (t), is called a basic, or fundamental set. Further, if we multiply each by an arbitrary constant and form the linear combinat ...
... exactly two independent solutions, say x1 (t) and x2 (t), meaning one is not a constant multiple of the other; they are not proportional. Such a set of solutions, x1 (t), x2 (t), is called a basic, or fundamental set. Further, if we multiply each by an arbitrary constant and form the linear combinat ...
Quaternions Multivariate Vectors
... temperature quantum computation is proven with respect to Marine Algae Photosynthetic harvesting of photons by chlorophyll. It allows us to postulate that it is ubiquitous to all plant life & to all animal life, too! For if the magnesium atom in chlorophyll is replaced with an iron atom, the result ...
... temperature quantum computation is proven with respect to Marine Algae Photosynthetic harvesting of photons by chlorophyll. It allows us to postulate that it is ubiquitous to all plant life & to all animal life, too! For if the magnesium atom in chlorophyll is replaced with an iron atom, the result ...
Hooke`s Law - UCSB Physics
... other ones. The most general solution to the differential equation is then given as a linear combination of these n “basis” solutions. In our case, it is clear that neither sine nor cosine can be written as a simple multiple of the other, and so these two solution are linearly independent. Because o ...
... other ones. The most general solution to the differential equation is then given as a linear combination of these n “basis” solutions. In our case, it is clear that neither sine nor cosine can be written as a simple multiple of the other, and so these two solution are linearly independent. Because o ...
A stochastic particle system modeling the Carleman equation
... Boltzmann-Grad limit when each molecule has very few collisions per unit time while it otherwise moves freely. When such a simplifying assumption is not fulfilled the analysis of the hydrodynamic behavior of the fluid becomes so intricate that no mathematically founded result is known and none seems ...
... Boltzmann-Grad limit when each molecule has very few collisions per unit time while it otherwise moves freely. When such a simplifying assumption is not fulfilled the analysis of the hydrodynamic behavior of the fluid becomes so intricate that no mathematically founded result is known and none seems ...
Intro to Physics - Fort Thomas Independent Schools
... Explain the difference between convergence and divergence as it relates to mirrors and lenses? Explain between reflection, refraction, diffraction and interference as it relates to waves (especially light)? Explain diffuse reflection? Explain how we see color? Explain dispersion and predict how whit ...
... Explain the difference between convergence and divergence as it relates to mirrors and lenses? Explain between reflection, refraction, diffraction and interference as it relates to waves (especially light)? Explain diffuse reflection? Explain how we see color? Explain dispersion and predict how whit ...
Wave packet
.gif?width=300)
In physics, a wave packet (or wave train) is a short ""burst"" or ""envelope"" of localized wave action that travels as a unit. A wave packet can be analyzed into, or can be synthesized from, an infinite set of component sinusoidal waves of different wavenumbers, with phases and amplitudes such that they interfere constructively only over a small region of space, and destructively elsewhere. Each component wave function, and hence the wave packet, are solutions of a wave equation. Depending on the wave equation, the wave packet's profile may remain constant (no dispersion, see figure) or it may change (dispersion) while propagating.Quantum mechanics ascribes a special significance to the wave packet; it is interpreted as a probability amplitude, its norm squared describing the probability density that a particle or particles in a particular state will be measured to have a given position or momentum. The wave equation is in this case the Schrödinger equation. It is possible to deduce the time evolution of a quantum mechanical system, similar to the process of the Hamiltonian formalism in classical mechanics. The dispersive character of solutions of the Schrödinger equation has played an important role in rejecting Schrödinger's original interpretation, and accepting the Born rule.In the coordinate representation of the wave (such as the Cartesian coordinate system), the position of the physical object's localized probability is specified by the position of the packet solution. Moreover, the narrower the spatial wave packet, and therefore the better localized the position of the wave packet, the larger the spread in the momentum of the wave. This trade-off between spread in position and spread in momentum is a characteristic feature of the Heisenberg uncertainty principle,and will be illustrated below.