RULES OF CHEMICAL NOMENCLATURE I. Elements (periodic
... B. Naming salts (other than binary) 1. name of metal plus name of ...
... B. Naming salts (other than binary) 1. name of metal plus name of ...
Unit 10
... Determine the types of reactants involved and the products formed in the reaction. Write down the correct formulae of reactants on the left hand side of the arrow. Write down the correct formulae of products on the right hand side of the arrow. Balance the equation with simple whole numbers such tha ...
... Determine the types of reactants involved and the products formed in the reaction. Write down the correct formulae of reactants on the left hand side of the arrow. Write down the correct formulae of products on the right hand side of the arrow. Balance the equation with simple whole numbers such tha ...
C07 Introduction to Chemical Reactions
... Kinetic means ____________________and the kinetic molecular theory (particle theory) states that all particles above ____________________________ are moving. Therefore, it is possible during this movement for two particles, molecules to come close enough, and collide with enough energy to create ...
... Kinetic means ____________________and the kinetic molecular theory (particle theory) states that all particles above ____________________________ are moving. Therefore, it is possible during this movement for two particles, molecules to come close enough, and collide with enough energy to create ...
Metals & Metallurgy
... Because the bands are so close, it is easy to promote electrons to a higher energy level. With M.O.'s, half of the orbitals are bonding and half are antibonding. Thus, with halffilled d subshells, the bonding M.O.'s are filled. After that, the electrons begin to fill antibonding orbitals which weake ...
... Because the bands are so close, it is easy to promote electrons to a higher energy level. With M.O.'s, half of the orbitals are bonding and half are antibonding. Thus, with halffilled d subshells, the bonding M.O.'s are filled. After that, the electrons begin to fill antibonding orbitals which weake ...
-6 -4 -2 0 2 -0.75 -0.50 -0.25 0 0.25 E/V vs. air i/m A cm 2% NO at
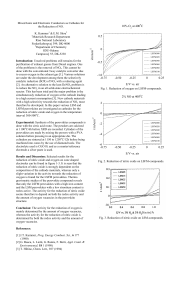
... machined into cones by the use of diamond tools. The electrolyte used is CGO10, and as a counter/reference electrode a silver paste is used. ...
... machined into cones by the use of diamond tools. The electrolyte used is CGO10, and as a counter/reference electrode a silver paste is used. ...
Semiconductors as catalysts for water splitting
... The spectral region depends on the band gap of the semi conductor Minimum band gap- 1.23 eV +(Thermodynamic losses (~0.4 eV)+ Over potentials required to ensure the fast reaction kinetics (~0.3-0.4eV)) So at least 1.9 eV, which corresponds to a wavelength of 650 nm Below 400 nm the intensity of sunl ...
... The spectral region depends on the band gap of the semi conductor Minimum band gap- 1.23 eV +(Thermodynamic losses (~0.4 eV)+ Over potentials required to ensure the fast reaction kinetics (~0.3-0.4eV)) So at least 1.9 eV, which corresponds to a wavelength of 650 nm Below 400 nm the intensity of sunl ...
Chemistry: The Study of Change
... elements of the bulk solid, and volatilization 挥发 of a solid by laser ablation, solar furnace, or some other method, followed by condensation of the volatilized components. The second category of nanoparticles fabrication methods involves condensation of atoms or molecules entities in a gas phase or ...
... elements of the bulk solid, and volatilization 挥发 of a solid by laser ablation, solar furnace, or some other method, followed by condensation of the volatilized components. The second category of nanoparticles fabrication methods involves condensation of atoms or molecules entities in a gas phase or ...
FLUID-SOLID SEPARATION_Adsorption
... Gas-phase adsorption 1) Removal of water from hydrocarbon gases 2) Sulphur compounds from natural gas 3) Solvent from air and other gases 4) Odor from air ...
... Gas-phase adsorption 1) Removal of water from hydrocarbon gases 2) Sulphur compounds from natural gas 3) Solvent from air and other gases 4) Odor from air ...