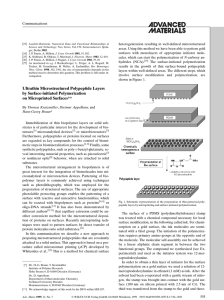
Spectroscopic Characterization of Mixed Fe−Ni
... coordination shells under electrochemical oxygen evolution conditions. Temperature programmed reaction spectroscopy showed the mixed oxide surfaces also have superior oxidation activity for methanol oxidation, and that the reactivity of the mixed oxide surface is substantially different than that of ...
... coordination shells under electrochemical oxygen evolution conditions. Temperature programmed reaction spectroscopy showed the mixed oxide surfaces also have superior oxidation activity for methanol oxidation, and that the reactivity of the mixed oxide surface is substantially different than that of ...
amcommu 555..558 - Leibniz-Institut für Polymerforschung Dresden
... the [FeIICrIII] derivative was twice that found in the XR4+ or [FeCp*2]+ analogues (203 mT compared with 110 mT). A much more profound effect on the magnetic properties is expected to occur when using mixtures of CrIII and FeIII in the construction of the anionic network, because competitive ferroma ...
... the [FeIICrIII] derivative was twice that found in the XR4+ or [FeCp*2]+ analogues (203 mT compared with 110 mT). A much more profound effect on the magnetic properties is expected to occur when using mixtures of CrIII and FeIII in the construction of the anionic network, because competitive ferroma ...
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Book-Abstracts - The Fritz Haber Center for Molecular dynamics
... possess known lifetimes which are often in the range of a few femtoseconds. The first property allows to selectively excite certain atoms of a system, for instance of an adsorbed molecule; the second property can be used as an internal clock to measure the rates of competing extremely fast processes ...
... possess known lifetimes which are often in the range of a few femtoseconds. The first property allows to selectively excite certain atoms of a system, for instance of an adsorbed molecule; the second property can be used as an internal clock to measure the rates of competing extremely fast processes ...
Coordination Chemistry of Life Processes: Bioinorganic Chemistry
... compounds NADPH and ATP, respectively, are used in the reduction or fixation of the carbon source, carbon dioxide. The biochemical processes involved in carbon dioxide fixation (Calvin Cycle) are not directly light dependent and are called 'dark reactions'. However, this stage uses a series of enzym ...
... compounds NADPH and ATP, respectively, are used in the reduction or fixation of the carbon source, carbon dioxide. The biochemical processes involved in carbon dioxide fixation (Calvin Cycle) are not directly light dependent and are called 'dark reactions'. However, this stage uses a series of enzym ...
Presentation
... of outer electrons, the positive (lose e-) variescharges +1 totend +7 (oxidation or negative Nonmetals to have numbers) can be predicted for negative oxidations single (monatomic) numbers. (gain atoms. e-) varies ...
... of outer electrons, the positive (lose e-) variescharges +1 totend +7 (oxidation or negative Nonmetals to have numbers) can be predicted for negative oxidations single (monatomic) numbers. (gain atoms. e-) varies ...
File
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
A1982NU66300001
... These were exciting times at University College London, with many new complexes being produced each week with the enthusiastic encouragementof Ron Nyholm. It was clear that there was a real need, in order to support this synthetic programme, for a rapid spectroscopic method for telling the likely ox ...
... These were exciting times at University College London, with many new complexes being produced each week with the enthusiastic encouragementof Ron Nyholm. It was clear that there was a real need, in order to support this synthetic programme, for a rapid spectroscopic method for telling the likely ox ...
Unit 1 Day 17 – Equipotential Surfaces
... • On a surface where V is constant, ΔV = 0, so either E = 0, or dl = 0, or cosθ = 0 (where cosθ is the angle between E & dl). For cosθ = 0, θ = 90°, E is d l • On a surface of a conductor of uniform charge, the entire surface is an equipotential surface, otherwise free electrons on the surface w ...
... • On a surface where V is constant, ΔV = 0, so either E = 0, or dl = 0, or cosθ = 0 (where cosθ is the angle between E & dl). For cosθ = 0, θ = 90°, E is d l • On a surface of a conductor of uniform charge, the entire surface is an equipotential surface, otherwise free electrons on the surface w ...
Structural Transformations of Zinc Oxide Layers on Pt(111)
... prepared at 300 K. When submonolayer amounts of Zn were deposited onto Pt(111) in 10−7 mbar of O2, aggregates of poorly defined nanoparticles dominated the surface (not shown here). In contrast, Zn deposition in vacuum onto the oxygenprecovered O(2 × 2)-Pt(111) surface [prepared by oxidation of the c ...
... prepared at 300 K. When submonolayer amounts of Zn were deposited onto Pt(111) in 10−7 mbar of O2, aggregates of poorly defined nanoparticles dominated the surface (not shown here). In contrast, Zn deposition in vacuum onto the oxygenprecovered O(2 × 2)-Pt(111) surface [prepared by oxidation of the c ...
Faculty of Science Department of chemistry Physical Chemistry (2)
... Apply kinetic treatments to complex reactions: chain, explosion, polymerization, and autocatalytic reaction. ...
... Apply kinetic treatments to complex reactions: chain, explosion, polymerization, and autocatalytic reaction. ...