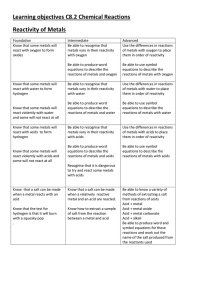
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... Know that metals react with oxygen to form basic oxides but that non metals react with oxygen to form acidic oxides and be able to use symbol equations to describe these ...
... Know that metals react with oxygen to form basic oxides but that non metals react with oxygen to form acidic oxides and be able to use symbol equations to describe these ...
Notes
... If iron is to rust the metal must come into contact with both oxygen and water. We can slow down the process and prevent rusting by using different methods of protection. The metal can be given physical protection to hinder corrosion. Physical protection means that air and water are prevented from r ...
... If iron is to rust the metal must come into contact with both oxygen and water. We can slow down the process and prevent rusting by using different methods of protection. The metal can be given physical protection to hinder corrosion. Physical protection means that air and water are prevented from r ...
Nitrogen`s oxidation states
... and then a to a deep red when stored under water for prolonged periods of time. The color change corresponds to a slow conversion to a more stable allotropic form, red phosphorus. Pure red phosphorus is polymeric material with a structure consisting of joined tetrahedra in which one of the six P—P b ...
... and then a to a deep red when stored under water for prolonged periods of time. The color change corresponds to a slow conversion to a more stable allotropic form, red phosphorus. Pure red phosphorus is polymeric material with a structure consisting of joined tetrahedra in which one of the six P—P b ...
Molecular Beam Epitaxy
... Growth of artificially layered crystals of various complexity with high degree of control and reproducibility In „low-dimensional structures“ the experimental physics based on quantum phenomena in brought to classroom Improved performance and new functionalities in heterojunction devices Materials e ...
... Growth of artificially layered crystals of various complexity with high degree of control and reproducibility In „low-dimensional structures“ the experimental physics based on quantum phenomena in brought to classroom Improved performance and new functionalities in heterojunction devices Materials e ...
Chapter 7
... • These are positively charged ions, resulting from a loss of an electron. • Metals tends to lose electrons from their highest occupied energy levels to become cations. This leaves a complete octet in the next-lowest energy level. • The charge for a cation is written as a number followed by a plus s ...
... • These are positively charged ions, resulting from a loss of an electron. • Metals tends to lose electrons from their highest occupied energy levels to become cations. This leaves a complete octet in the next-lowest energy level. • The charge for a cation is written as a number followed by a plus s ...
Trends in the band structures of the group I and II oxides
... The difference in arrival times at the electron detectors for correlated pairs will always be the same, whereas random pairs have random arrival times. Collecting data for a large number of collisions over a range of energy and momentum values directly maps the probability density of the target elec ...
... The difference in arrival times at the electron detectors for correlated pairs will always be the same, whereas random pairs have random arrival times. Collecting data for a large number of collisions over a range of energy and momentum values directly maps the probability density of the target elec ...
Unit 4 - Dorman High School
... If the difference in electronegativity is large enough the bond between the atoms will have polarity. What is this called? Any diatomic molecule will have a dipole moment. When will they not? Some polyatomic molecules can have dipole moments. When will this occur? IV. Stable Electron Configurations ...
... If the difference in electronegativity is large enough the bond between the atoms will have polarity. What is this called? Any diatomic molecule will have a dipole moment. When will they not? Some polyatomic molecules can have dipole moments. When will this occur? IV. Stable Electron Configurations ...
E ref (W)
... function of incidentenergy in the in the range from 0.5eV to 200eV. Mean range and projected range distribution of C with different incident energy on tungsten surface are discussed. Vacancy formation energy and the migration energy, C interstitial and substitutional formation energies have been c ...
... function of incidentenergy in the in the range from 0.5eV to 200eV. Mean range and projected range distribution of C with different incident energy on tungsten surface are discussed. Vacancy formation energy and the migration energy, C interstitial and substitutional formation energies have been c ...
Chapter 2
... Atoms or groups of atoms with a charge. Cations- positive ions - get by losing electrons(s). Anions- negative ions - get by gaining electron(s). Ionic bonding- held together by the opposite ...
... Atoms or groups of atoms with a charge. Cations- positive ions - get by losing electrons(s). Anions- negative ions - get by gaining electron(s). Ionic bonding- held together by the opposite ...