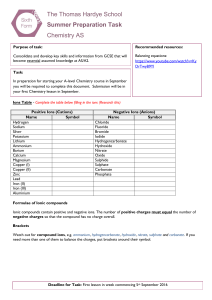
Pre-Board Examination2016 Class : XII MM: 70 Subject : Chemistry
... (b) Out of C and CO, which is a better reducing agent at the lower temperature range in the blast furnace to extract iron from the oxide ore? 13. (a) Which metal in the first transition series (3d series) exhibits +1 oxidation state most frequently and why? (b) Which of the following cations are col ...
... (b) Out of C and CO, which is a better reducing agent at the lower temperature range in the blast furnace to extract iron from the oxide ore? 13. (a) Which metal in the first transition series (3d series) exhibits +1 oxidation state most frequently and why? (b) Which of the following cations are col ...
The Periodic Table Trends
... When atoms get close to each other, the nucleus of one atom will interact with the other atom’s valence electrons. A chemical reaction and bond can result from that interaction. ...
... When atoms get close to each other, the nucleus of one atom will interact with the other atom’s valence electrons. A chemical reaction and bond can result from that interaction. ...
Big Idea #3
... When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) © 2009, Prentice-Hall, Inc. ...
... When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) © 2009, Prentice-Hall, Inc. ...
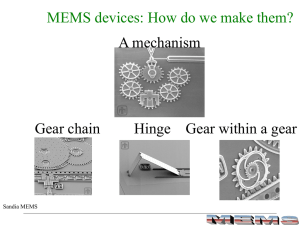
MEMS Processing
... - Amorphous/columnar grained structures: Compressive stress - Equiaxed grained structures: Tensile stress - Thick films have less stress than thinner films -ANNEALING CAN REDUCE STRESSES BY A FACTOR OF 10-100 ...
... - Amorphous/columnar grained structures: Compressive stress - Equiaxed grained structures: Tensile stress - Thick films have less stress than thinner films -ANNEALING CAN REDUCE STRESSES BY A FACTOR OF 10-100 ...
When Gold Is Not Noble: Nanoscale Gold
... LHt. Through mapping of the potential energy surface along the C-O1 reaction coordinate (via total relaxation of the system with the variable C-O1 distance as a constraint) we determined a rather low energy barrier ∆Eb (LHt) ) 0.1 eV (occurring at db(CO1) ≈ 2.0 Å) for the LHT oxidation channel with ...
... LHt. Through mapping of the potential energy surface along the C-O1 reaction coordinate (via total relaxation of the system with the variable C-O1 distance as a constraint) we determined a rather low energy barrier ∆Eb (LHt) ) 0.1 eV (occurring at db(CO1) ≈ 2.0 Å) for the LHT oxidation channel with ...
Kinetics of Oxygen Reduction in Aprotic Li–O2 Cells: A Model
... discharge (by)products.29,30 The system dynamics is neglected and the model only addresses the steady-state behavior. This is especially a valid assumption in the case of carbon-based cathodes where the potential rapidly levels off toward a plateau over a wide range of current densities. We note that ...
... discharge (by)products.29,30 The system dynamics is neglected and the model only addresses the steady-state behavior. This is especially a valid assumption in the case of carbon-based cathodes where the potential rapidly levels off toward a plateau over a wide range of current densities. We note that ...
Studies of some structural and transition metal complexes of
... and anilides are found to be very good complexing agents especially for transition and inner transition metal ions (Basheir et al. 1988).Their complexing ability is enhanced by introduction of active groups like oximes, hydrazones, thiosemicarbazones etc. The combination products of these two classe ...
... and anilides are found to be very good complexing agents especially for transition and inner transition metal ions (Basheir et al. 1988).Their complexing ability is enhanced by introduction of active groups like oximes, hydrazones, thiosemicarbazones etc. The combination products of these two classe ...
Chapter
... interactions. The aqueous solvent in the pulp system is divided between two subvolumes: The volume of water surrounding the fibres and the volume of water inside the fibre walls. The amount of water enclosed by the fibre walls depends on the type of pulp and to some extent the chemical composition o ...
... interactions. The aqueous solvent in the pulp system is divided between two subvolumes: The volume of water surrounding the fibres and the volume of water inside the fibre walls. The amount of water enclosed by the fibre walls depends on the type of pulp and to some extent the chemical composition o ...
AAN025_V1 Interfacial rheometry
... measured. Note that for a mica plate a zero contact angle is assumed. Any contamination of the mica Adsorption and orientation plate has to be removed by flaming before the Amphiphilic materials are adsorbed at the interexperiment face to form an orientated monomolecular layer The pendant drop metho ...
... measured. Note that for a mica plate a zero contact angle is assumed. Any contamination of the mica Adsorption and orientation plate has to be removed by flaming before the Amphiphilic materials are adsorbed at the interexperiment face to form an orientated monomolecular layer The pendant drop metho ...
ST. PAUL`S CONVENT SCHOOL METALLIC RAINBOW 金屬彩虹
... In part I, NH3, en, citrate and glycine were chosen for Cu2+ for the conduction of colorimetry; EDTA, oxalate and tartrate for Ni2+; conc. HCl, en and tartrate for Co2+; NaBr, EDTA and leucine for Cr3+; KSCN, rust indicator, en, citrate and glycine for Fe3+. In part II, en and glycine were selec ...
... In part I, NH3, en, citrate and glycine were chosen for Cu2+ for the conduction of colorimetry; EDTA, oxalate and tartrate for Ni2+; conc. HCl, en and tartrate for Co2+; NaBr, EDTA and leucine for Cr3+; KSCN, rust indicator, en, citrate and glycine for Fe3+. In part II, en and glycine were selec ...