pdf
... and colleagues2 now report independent experiments in which striking effects of Pauli exclusion are observed in dilute gases of atoms cooled down to less than a millionth of a degree above absolute zero. These atomic clouds, 1011 times larger than atomic nuclei and 1011 times smaller than white dwar ...
... and colleagues2 now report independent experiments in which striking effects of Pauli exclusion are observed in dilute gases of atoms cooled down to less than a millionth of a degree above absolute zero. These atomic clouds, 1011 times larger than atomic nuclei and 1011 times smaller than white dwar ...
Nuclear ppt notes
... Heavier elements need more neutrons than protons to hold the nucleus together. ...
... Heavier elements need more neutrons than protons to hold the nucleus together. ...
Chapter 4 Assignment Answers
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
Chapter 29
... Energy in a Fission Process Binding energy for heavy nuclei is about 7.2 MeV per nucleon Binding energy for intermediate nuclei is about 8.2 MeV per nucleon Therefore, the fission fragments have less mass than the nucleons in the original nuclei This decrease in mass per nucleon appears as ...
... Energy in a Fission Process Binding energy for heavy nuclei is about 7.2 MeV per nucleon Binding energy for intermediate nuclei is about 8.2 MeV per nucleon Therefore, the fission fragments have less mass than the nucleons in the original nuclei This decrease in mass per nucleon appears as ...
INTRODUCTION TO ELEMENTARY PARTICLE PHYSICS
... behavior have nothing to do with the detailed form of the interactions. Instead they follow directly from relativity, from quantum mechanics, or from the combination of the two. For example, in relativity, energy and momentum are always conserved, but (rest) mass is not. Thus the decay A p + A is pe ...
... behavior have nothing to do with the detailed form of the interactions. Instead they follow directly from relativity, from quantum mechanics, or from the combination of the two. For example, in relativity, energy and momentum are always conserved, but (rest) mass is not. Thus the decay A p + A is pe ...
33 Atomic Nucleus and Radioactivity Answers and Solutions for
... charge (positive)as the target atomic nuclei, the protons must be driven into the target area with enormous energies if they are to bombard the nuclei. Lower-energy protons would be easily electrically repelled by any nuclei they approach. ...
... charge (positive)as the target atomic nuclei, the protons must be driven into the target area with enormous energies if they are to bombard the nuclei. Lower-energy protons would be easily electrically repelled by any nuclei they approach. ...
32 The Atom and the Quantum Answers and Solutions for Chapter
... It is the wave nature of matter that keeps atoms apart and gives bulk to matter in the world around us. Otherwise everything would collapse and there would be no matter as we know it. ...
... It is the wave nature of matter that keeps atoms apart and gives bulk to matter in the world around us. Otherwise everything would collapse and there would be no matter as we know it. ...
Modern Physics P age | 1 AP Physics B
... 13. The scattering of alpha particles by a thin gold foil was measured by Geiger and Marsden. The Rutherford model of the atom was proposed in order to explain why (A) more particles scattered through angles greater than 90° than through angles less than 90° (B) the fraction of particles scattered t ...
... 13. The scattering of alpha particles by a thin gold foil was measured by Geiger and Marsden. The Rutherford model of the atom was proposed in order to explain why (A) more particles scattered through angles greater than 90° than through angles less than 90° (B) the fraction of particles scattered t ...
Rusov-Presentation-Sofia-Mateev-NuclearFission
... Kramers description of the alpha decay, cluster radioactivity and spontaneous fission, which was based on the newest experimental data for alpha-decay of even-even super heavy nuclei (Z=114, 116, 118) have shown the good coincidence of the experimental and theoretical half-life depend on of alphadec ...
... Kramers description of the alpha decay, cluster radioactivity and spontaneous fission, which was based on the newest experimental data for alpha-decay of even-even super heavy nuclei (Z=114, 116, 118) have shown the good coincidence of the experimental and theoretical half-life depend on of alphadec ...
What is a Force?
... This particle would be holding not only protons to protons but protons to neutrons and neutrons to neutrons He predicted the properties the new particle should have. The neutral pion (π0) was discovered in 1947 and it was thought to be totally responsible for the strong force. We now know this is no ...
... This particle would be holding not only protons to protons but protons to neutrons and neutrons to neutrons He predicted the properties the new particle should have. The neutral pion (π0) was discovered in 1947 and it was thought to be totally responsible for the strong force. We now know this is no ...
particlephysics
... Another reason why neutrino’s needed to exist… Conservation of energy • Experimentally β particle energy varied – problem as decay releases fixed amount • (anti) neutrino has energy left over ...
... Another reason why neutrino’s needed to exist… Conservation of energy • Experimentally β particle energy varied – problem as decay releases fixed amount • (anti) neutrino has energy left over ...
Problem Set 7
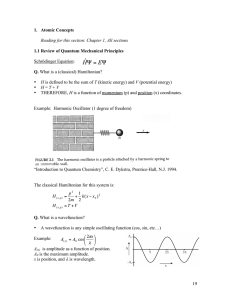
... The Lyman series is a particular set of spectral lines of the Hydrogen atom. When the Hydrogen atom de-excites from an excited state with principal number ni to a final state with n = 1, it emits a photon in the Lyman series, of wavelength ...
... The Lyman series is a particular set of spectral lines of the Hydrogen atom. When the Hydrogen atom de-excites from an excited state with principal number ni to a final state with n = 1, it emits a photon in the Lyman series, of wavelength ...