Conservation of the metabolomic response to starvation across two divergent microbes.
... same metabolic network (2) despite radically different compartmentation. The concentrations and fluxes of metabolites depend on the interactions among this conserved network structure, the cellular environment, and species-specific factors, such as the location, activities, and regulation of metabol ...
... same metabolic network (2) despite radically different compartmentation. The concentrations and fluxes of metabolites depend on the interactions among this conserved network structure, the cellular environment, and species-specific factors, such as the location, activities, and regulation of metabol ...
Conservation of the metabolomic response to starvation across two divergent microbes.
... same metabolic network (2) despite radically different compartmentation. The concentrations and fluxes of metabolites depend on the interactions among this conserved network structure, the cellular environment, and species-specific factors, such as the location, activities, and regulation of metabol ...
... same metabolic network (2) despite radically different compartmentation. The concentrations and fluxes of metabolites depend on the interactions among this conserved network structure, the cellular environment, and species-specific factors, such as the location, activities, and regulation of metabol ...
Chapter 25
... The novel prosthetic groups of nitrate reductase and nitrite reductase. (a) The molybdenum cofactor of nitrate reductase. The molybdenum-free version of this compound is a pterin derivative called molybdopterin. (b) Siroheme, a uroporphyrin derivative, is a member of the isobacteriochlorin class of ...
... The novel prosthetic groups of nitrate reductase and nitrite reductase. (a) The molybdenum cofactor of nitrate reductase. The molybdenum-free version of this compound is a pterin derivative called molybdopterin. (b) Siroheme, a uroporphyrin derivative, is a member of the isobacteriochlorin class of ...
Problem Set 1 - Andrew.cmu.edu
... in the ammonium ion, NH4+. Nitrogen has 7 electrons. Two of these are in the 1st shell and the remaining five are in the second shell. The second shell contains four orbitals, three of these will have a single electron (that’s why nitrogen forms three bonds). The fourth is full, with two electrons. ...
... in the ammonium ion, NH4+. Nitrogen has 7 electrons. Two of these are in the 1st shell and the remaining five are in the second shell. The second shell contains four orbitals, three of these will have a single electron (that’s why nitrogen forms three bonds). The fourth is full, with two electrons. ...
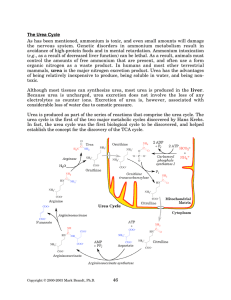
The Urea Cycle - Rose
... The urea cycle is a series of reactions that converts toxic ammonium into the nontoxic nitrogen excretion production urea. The urea cycle requires ornithine as a carbon backbone, and aspartate and free ammonium as nitrogen donors. The free ammonium used in the urea cycle is largely released from glu ...
... The urea cycle is a series of reactions that converts toxic ammonium into the nontoxic nitrogen excretion production urea. The urea cycle requires ornithine as a carbon backbone, and aspartate and free ammonium as nitrogen donors. The free ammonium used in the urea cycle is largely released from glu ...
Name - TeacherWeb
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
... The elements in Group 18 are known as the noble gases. They do not usually form compounds because they do not like to gain, lose, or share electrons. All of the noble gases exist in the Earth’s atmosphere, but only in small amounts. ...
1 Introduction
... degradation is deamination, a process whereby the amino group is removed and that is catalysed by enzymes called transaminases. The removal of the amino group begins with the transfer of the amino group to alpha-ketoglutarate. The product of this transfer is an alpha-keto acid formed from the amino ...
... degradation is deamination, a process whereby the amino group is removed and that is catalysed by enzymes called transaminases. The removal of the amino group begins with the transfer of the amino group to alpha-ketoglutarate. The product of this transfer is an alpha-keto acid formed from the amino ...
Amino acid catabolism
... glutamine respectively forming non-toxic compounds that are excreted in urine. Thus the body runs low in glycine and glutamine and starts synthasizing these AA using the ammonia available in system. Thus clearing the system of excess ammonia. ...
... glutamine respectively forming non-toxic compounds that are excreted in urine. Thus the body runs low in glycine and glutamine and starts synthasizing these AA using the ammonia available in system. Thus clearing the system of excess ammonia. ...
Theme 2 Implementation
... foraminifera, pteropods, corals, and bivalves Physiological responses of marine calcifiers to ocean acidification at the species and community levels using genomic and proteomic approaches Rates of genetic change in calcifying genes in key calcifiers from decadal to geological time scales Effect of ...
... foraminifera, pteropods, corals, and bivalves Physiological responses of marine calcifiers to ocean acidification at the species and community levels using genomic and proteomic approaches Rates of genetic change in calcifying genes in key calcifiers from decadal to geological time scales Effect of ...
removal of amino gp from glutamate to release ammonia Other
... Transamination: Transfer of amino group to a-ketoglutarate. There are several aminotransferases specific to different amino acids. In this step amino group from all the amino acids are transferred to a-ketoglutarate and they exist as glutamate. ...
... Transamination: Transfer of amino group to a-ketoglutarate. There are several aminotransferases specific to different amino acids. In this step amino group from all the amino acids are transferred to a-ketoglutarate and they exist as glutamate. ...
Chapter 37 – Plant Nutrition
... 1. The metabolism of soil bacteria makes nitrogen available to plants. ...
... 1. The metabolism of soil bacteria makes nitrogen available to plants. ...
Lecture 10 - Protein Turnover and Amino Acid
... cancers are associtated with this type of activity. ...
... cancers are associtated with this type of activity. ...
Chemistry 2000 Lecture 20: Organic bases
... through an inductive effect: alkyl groups are more polarizable than hydrogen, so they are better at stabilizing the positive charge of the acid. 2. The acid form is stabilized by hydrogen bonding to water. Increasing the number of alkyl substituents decreases the number of hydrogen bonds that can be ...
... through an inductive effect: alkyl groups are more polarizable than hydrogen, so they are better at stabilizing the positive charge of the acid. 2. The acid form is stabilized by hydrogen bonding to water. Increasing the number of alkyl substituents decreases the number of hydrogen bonds that can be ...
Ecology Unit Study Guide Levels of organization Organism
... CO2 in the atmosphere, increase global warming rates and increase the amount of acid rain. ...
... CO2 in the atmosphere, increase global warming rates and increase the amount of acid rain. ...
V. How is matter cycled?
... - Nitrogen is needed to make chlorophyll in plants, which is needed for plants to go through photosynthesis. ...
... - Nitrogen is needed to make chlorophyll in plants, which is needed for plants to go through photosynthesis. ...
No Slide Title
... • Nitrogen-fixing bacteria living in the root nodules of leguminous plants: - The bacteria obtain carbohydrates from the ...
... • Nitrogen-fixing bacteria living in the root nodules of leguminous plants: - The bacteria obtain carbohydrates from the ...
ecology - cloudfront.net
... * nutrients: chemical substances an organism needs to build tissues & carry out essential life fxns A. carbon cycle (fig 3-13) – C stored in: air as CO2 & methane (CH4) oceans as dissolved CO2 land as fossil fuels & rocks B. nitrogen cycle (N used to build AA) (fig 3-14) nitrogen fixation – ...
... * nutrients: chemical substances an organism needs to build tissues & carry out essential life fxns A. carbon cycle (fig 3-13) – C stored in: air as CO2 & methane (CH4) oceans as dissolved CO2 land as fossil fuels & rocks B. nitrogen cycle (N used to build AA) (fig 3-14) nitrogen fixation – ...
Organic Matter
... A typical farmer is doing a "good job" of soil conservation if he loses less than 1/16" of topsoil each year. How many pounds of soil does he/she lose each year ...
... A typical farmer is doing a "good job" of soil conservation if he loses less than 1/16" of topsoil each year. How many pounds of soil does he/she lose each year ...
Nitrogen Balance and Protein Requirements: Definition and
... the body to synthesise them, nitrogen will be needed for their de novo synthesis. This nitrogen in turn must be derived either from EAA catabolism (thus increasing their requirement above theoretical values) or from the diet. In this respect, although NEAA can theoretically be replaced, they are req ...
... the body to synthesise them, nitrogen will be needed for their de novo synthesis. This nitrogen in turn must be derived either from EAA catabolism (thus increasing their requirement above theoretical values) or from the diet. In this respect, although NEAA can theoretically be replaced, they are req ...
Protein mteabolism
... glutamate (using NAD or NADP) and catalyze the reversal reduction reaction using NADH (or NADPH) ...
... glutamate (using NAD or NADP) and catalyze the reversal reduction reaction using NADH (or NADPH) ...
Nitrogen cycle

The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms. This transformation can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is nitrogen, making it the largest pool of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitrogen cycle.