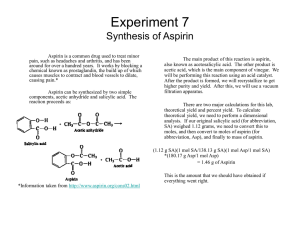
Identification and determination of pI of amino acids
... poorly developed blood-brain barrier are exposed to toxic metabolites and threaten serious damage of development and function of neural tissue. In other cases, there is a lack of transporter for absorption or excretion of amino acids. There is a plenty of disorders of ureosynthetic cycle. Absence or ...
... poorly developed blood-brain barrier are exposed to toxic metabolites and threaten serious damage of development and function of neural tissue. In other cases, there is a lack of transporter for absorption or excretion of amino acids. There is a plenty of disorders of ureosynthetic cycle. Absence or ...
Chem Reactions (and Balancing Equations)
... • Two things replace each other. • Reactants must be two ionic compounds or acids. • Usually in aqueous solution AB + CD AD + CB ...
... • Two things replace each other. • Reactants must be two ionic compounds or acids. • Usually in aqueous solution AB + CD AD + CB ...
Acid‒base reaction
... (In modern times, the use of H+ is regarded as a shorthand for H3O+, since it is now known that the bare proton H+ does not exist as a free species in solution.) This leads to the definition that in Arrhenius acid–base reactions, a salt and water is formed from the reaction between an acid and a bas ...
... (In modern times, the use of H+ is regarded as a shorthand for H3O+, since it is now known that the bare proton H+ does not exist as a free species in solution.) This leads to the definition that in Arrhenius acid–base reactions, a salt and water is formed from the reaction between an acid and a bas ...
summer fun - West Windsor-Plainsboro Regional School District
... electrolyte which normally decomposes into NH3 (g) and HOH (l). Technically speaking, the pure compound ammonium hydroxide has never been isolated and the substance is more correctly known as aqueous ammonia. Most other hydroxides are insoluble. Pure liquid hydroxides are strong hydroxides because t ...
... electrolyte which normally decomposes into NH3 (g) and HOH (l). Technically speaking, the pure compound ammonium hydroxide has never been isolated and the substance is more correctly known as aqueous ammonia. Most other hydroxides are insoluble. Pure liquid hydroxides are strong hydroxides because t ...
X273/13/02
... 17. Iodide ions are oxidised by acidified nitrite ions according to the equation 2NO2– + 2I– + 4H+ → 2NO + I2 + 2H2O Addition of sodium ethanoate to the reaction mixture slows down the formation of iodine. The most likely explanation for this effect is ...
... 17. Iodide ions are oxidised by acidified nitrite ions according to the equation 2NO2– + 2I– + 4H+ → 2NO + I2 + 2H2O Addition of sodium ethanoate to the reaction mixture slows down the formation of iodine. The most likely explanation for this effect is ...
No Slide Title - McMaster Chemistry
... conjugate BASE are both present at the same time WEAK ACID: (acetic acid a.k.a. vinegar) CH3CO2H + H2O CH3CO2- (aq) + H3O+ (aq) WEAK BASE: NH3 (g) + H2O NH4+ (aq) + OH- (aq) 1A03/1E03 Types of Reactions (2) ...
... conjugate BASE are both present at the same time WEAK ACID: (acetic acid a.k.a. vinegar) CH3CO2H + H2O CH3CO2- (aq) + H3O+ (aq) WEAK BASE: NH3 (g) + H2O NH4+ (aq) + OH- (aq) 1A03/1E03 Types of Reactions (2) ...
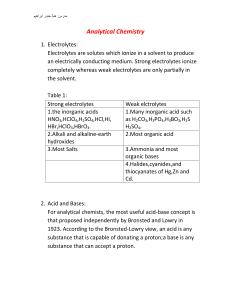
Analytical Chemistry
... The molecular formula may additionally provide structural information thus, C2H6O is both the empirical and the chemical formula for the chemically ferent ethanol , C2H5OH, and dimethyl ether CH3OCH3 . 5. The mole: It is gram formula weight per formula weight (M.wt.,which is the summation of the ato ...
... The molecular formula may additionally provide structural information thus, C2H6O is both the empirical and the chemical formula for the chemically ferent ethanol , C2H5OH, and dimethyl ether CH3OCH3 . 5. The mole: It is gram formula weight per formula weight (M.wt.,which is the summation of the ato ...
8 - THE DETERMINATION OF THE CONCENTRATION
... you will use apply to the analysis of any diprotic acid, you are likely to encounter the acidity of amino acids more frequently in molecular life science than any other type of compound. While the R groups may contain acidic or basic groups, much of the chemistry of the amino acids results from the ...
... you will use apply to the analysis of any diprotic acid, you are likely to encounter the acidity of amino acids more frequently in molecular life science than any other type of compound. While the R groups may contain acidic or basic groups, much of the chemistry of the amino acids results from the ...
equilibrium questions - Southington Public Schools
... (d) Calculate the number of moles of NaOBr(s) that would have to be added to 125 mL of 0.160 M HOBr to –9 M. Assume that volume change is negligible. produce a buffer solution with [H+] = 5.00 (e) HOBr is a weaker acid than HBrO3. Account for this fact in terms of molecular structure. 2001 A Answer ...
... (d) Calculate the number of moles of NaOBr(s) that would have to be added to 125 mL of 0.160 M HOBr to –9 M. Assume that volume change is negligible. produce a buffer solution with [H+] = 5.00 (e) HOBr is a weaker acid than HBrO3. Account for this fact in terms of molecular structure. 2001 A Answer ...
activity series
... It is most important for a chemist to be able to write correctly balanced equations and to interpret equations written by others. It is also very helpful if he/she knows how to predict the products of certain specific types of reactions. ...
... It is most important for a chemist to be able to write correctly balanced equations and to interpret equations written by others. It is also very helpful if he/she knows how to predict the products of certain specific types of reactions. ...
AP CHEMISTRY - An Incomplete List of Topics
... Write equilibrium expressions Kc, Kp, Ka, Kb, Ksp Know when prods or reacts are favored. Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temp change. Be able to use H in le Chatelier's principle and K. Solve I.C.E. ...
... Write equilibrium expressions Kc, Kp, Ka, Kb, Ksp Know when prods or reacts are favored. Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temp change. Be able to use H in le Chatelier's principle and K. Solve I.C.E. ...
Lecture 22 - Chemistry Courses
... Acid-base equilibria in water The pH of acids and bases Acid-base titrations The pH curve Buffers Indicators ...
... Acid-base equilibria in water The pH of acids and bases Acid-base titrations The pH curve Buffers Indicators ...
Various Types of RXNS
... NOTE: Not all of the following chemical reactions are balanced. They have been intentionally left unbalanced for your practice. ...
... NOTE: Not all of the following chemical reactions are balanced. They have been intentionally left unbalanced for your practice. ...
Chemistry- CST Review
... 2. What factors increase the rate (speed) of a reaction? (Like concentration, pressure, and temperature) The increase of concentration of reactants, pressure, and temperature are factors that increase reaction rate. 3. What does a catalyst do to the reaction rate? A catalyst decreases the activation ...
... 2. What factors increase the rate (speed) of a reaction? (Like concentration, pressure, and temperature) The increase of concentration of reactants, pressure, and temperature are factors that increase reaction rate. 3. What does a catalyst do to the reaction rate? A catalyst decreases the activation ...
Chemistry 1A Final Exam December 12, 2001 Page 1 of 16 (Closed
... Instructions: Enter answers in the boxes where provided. Show all work for which you wish to receive credit. Where explanations are required, only the first fifteen words will be considered ...
... Instructions: Enter answers in the boxes where provided. Show all work for which you wish to receive credit. Where explanations are required, only the first fifteen words will be considered ...
CHEMISTRY: Practice Spring Final
... Note: Do not JUST study this practice exam; it does not contain every topic that may appear on your final exam. Be sure to look at your review guide to see a list of topics you are responsible for. Also, this practice test is broken up by topic; your final exam will not be. ...
... Note: Do not JUST study this practice exam; it does not contain every topic that may appear on your final exam. Be sure to look at your review guide to see a list of topics you are responsible for. Also, this practice test is broken up by topic; your final exam will not be. ...
File
... It’s time to practice what you have already learned about moles, chemical reactions and dimensional analysis. We will learn one new conversion factor and then combine it with other concepts. Molar Volume is the volume of one mole of gas. Since the space between molecules in a gas is very great compa ...
... It’s time to practice what you have already learned about moles, chemical reactions and dimensional analysis. We will learn one new conversion factor and then combine it with other concepts. Molar Volume is the volume of one mole of gas. Since the space between molecules in a gas is very great compa ...