Organic chemistry
... if there is enough chlorine present. As each remaining hydrogen atom is removed from CH3Cl and replaced by a chlorine atom, a mixture of CH2Cl2, CHCl3 and CCl4 is formed. ...
... if there is enough chlorine present. As each remaining hydrogen atom is removed from CH3Cl and replaced by a chlorine atom, a mixture of CH2Cl2, CHCl3 and CCl4 is formed. ...
04_Label_Edit_Images
... component of DNA that has been modified by addition of the methyl group. Addition of a methyl group to DNA, or to molecules bound to DNA, affects expression of genes. Arrangement of methyl groups in male and female ...
... component of DNA that has been modified by addition of the methyl group. Addition of a methyl group to DNA, or to molecules bound to DNA, affects expression of genes. Arrangement of methyl groups in male and female ...
AMINES
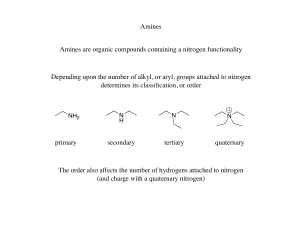
... has more than R3NH+ .Moreover larger and more number of alkyl groups also cause steric hindrance to formation of H bonds with water. Q13) The order of base strength in aqueous solution for ethyl substituted amines is in this order (C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 while for methyl substituted ami ...
... has more than R3NH+ .Moreover larger and more number of alkyl groups also cause steric hindrance to formation of H bonds with water. Q13) The order of base strength in aqueous solution for ethyl substituted amines is in this order (C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 while for methyl substituted ami ...
Back
... (b) How is organic chemistry defined nowadays? (b) Nowadays, scientists have discovered that many organic compounds can be synthesized from inorganic substances. The updated definition of organic chemistry is the study of carbon compounds, except for carbon monoxide, carbon dioxide, carbonates, hydr ...
... (b) How is organic chemistry defined nowadays? (b) Nowadays, scientists have discovered that many organic compounds can be synthesized from inorganic substances. The updated definition of organic chemistry is the study of carbon compounds, except for carbon monoxide, carbon dioxide, carbonates, hydr ...
226 amines lec
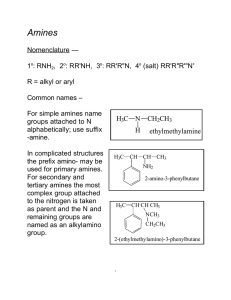
... -NH2 is considered a substituent, like chloro or nitro, and is called amino. It is located by number in the usual way. For secondary and tertiary amines, the largest alkyl group is taken as the ...
... -NH2 is considered a substituent, like chloro or nitro, and is called amino. It is located by number in the usual way. For secondary and tertiary amines, the largest alkyl group is taken as the ...
Chapter 25 Organic and Biological Chemistry
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
STUDY GUIDE
... MAIN IDEA: An alkene is a hydrocarbon that has at least one double bond between two carbon atoms. The carbon chain is numbered using the lowest number for the double bond. The root name ends in -ene. An alkyne is a hydrocarbon that has at least one triple bond between two carbon atoms. Naming alkyn ...
... MAIN IDEA: An alkene is a hydrocarbon that has at least one double bond between two carbon atoms. The carbon chain is numbered using the lowest number for the double bond. The root name ends in -ene. An alkyne is a hydrocarbon that has at least one triple bond between two carbon atoms. Naming alkyn ...
Chemistry Chapter 18 - Pearson Schools and FE Colleges
... Section D: Organic Chemistry When you start doing organic chemistry, you are suddenly faced with a whole lot of new compounds with strange names and unfamiliar ways of drawing them. It can be quite scary! ...
... Section D: Organic Chemistry When you start doing organic chemistry, you are suddenly faced with a whole lot of new compounds with strange names and unfamiliar ways of drawing them. It can be quite scary! ...
Organic Chemistry
... Arenes, also called aromatic hydrocarbons, are compounds that contain a benzene ring, a six-member carbon ring of alternating single and double carbon–carbon bonds. This class of compounds was named aromatic because many of them have strong and distinctive odors. Now this term refers to a class of c ...
... Arenes, also called aromatic hydrocarbons, are compounds that contain a benzene ring, a six-member carbon ring of alternating single and double carbon–carbon bonds. This class of compounds was named aromatic because many of them have strong and distinctive odors. Now this term refers to a class of c ...
Acylation of aromatic alcohols and phenols over InCl3
... from 7 to 96% on increasing the InCl3 loading from zero to 20%. The results clearly show that InCl3 (20%)/Mont. K-10 is a much superior catalyst than the Mont. K-10 without InCl3. It may be noted that use of InCl3 as a catalyst has also been reported earlier in a number of other other organic reacti ...
... from 7 to 96% on increasing the InCl3 loading from zero to 20%. The results clearly show that InCl3 (20%)/Mont. K-10 is a much superior catalyst than the Mont. K-10 without InCl3. It may be noted that use of InCl3 as a catalyst has also been reported earlier in a number of other other organic reacti ...
Chapter 19
... The amide does not generally react further because the lone pair of electrons is delocalized ...
... The amide does not generally react further because the lone pair of electrons is delocalized ...
Chapter 13 – Organic Chemistry
... theory (Chapter 8.6), that electronic energy levels get closer together as electrons become spread out over many bonds. Also recall from Chapter 6.5 that pi electrons can be delocalized over many carbon atoms. Thus, while most organic compounds are white or colorless because their electronic energy ...
... theory (Chapter 8.6), that electronic energy levels get closer together as electrons become spread out over many bonds. Also recall from Chapter 6.5 that pi electrons can be delocalized over many carbon atoms. Thus, while most organic compounds are white or colorless because their electronic energy ...
1 - Rosshall Academy
... Name the straight chain alkanes C1 to C8 from molecular formulae, shortened and full structural formulae. Write molecular formulae and draw full and shortened structural formulae given the names of straight-chain alkanes C1 to C8 Give the systematic names of branched chain alkanes from shortened and ...
... Name the straight chain alkanes C1 to C8 from molecular formulae, shortened and full structural formulae. Write molecular formulae and draw full and shortened structural formulae given the names of straight-chain alkanes C1 to C8 Give the systematic names of branched chain alkanes from shortened and ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.