Evolution Evidence
... These are called homologous structures, and tend to be common in organisms which are related If the similarity is not apparent in the adult versions of an organism, then it may be necessary to observe the embryonic structure ...
... These are called homologous structures, and tend to be common in organisms which are related If the similarity is not apparent in the adult versions of an organism, then it may be necessary to observe the embryonic structure ...
Lect4 Proteins
... 2. Give the characteristics of each amino acid in the polypeptide chain. 3. How long is the original RNA sequence and how long is the protein sequence? ...
... 2. Give the characteristics of each amino acid in the polypeptide chain. 3. How long is the original RNA sequence and how long is the protein sequence? ...
Functional Groups and Macromolecules
... fused ring structures – Cholesterol is an example of a steroid that plays a ...
... fused ring structures – Cholesterol is an example of a steroid that plays a ...
1. I can tell the difference between mRNA, tRNA, and rRNA
... RNA polymerase is the enzyme responsible for transcription. It then unwinds and separates the DNA and then adds complementary RNA nucleotides using the DNA as a pattern. Once the gene is fully transcribed into RNA, the mRNA is edited. ...
... RNA polymerase is the enzyme responsible for transcription. It then unwinds and separates the DNA and then adds complementary RNA nucleotides using the DNA as a pattern. Once the gene is fully transcribed into RNA, the mRNA is edited. ...
Chapter 3 Biological Molecules
... Primary structure is the sequence of amino acids linked together in a protein Secondary structures are helices and pleated sheets Tertiary structure refers to complex foldings of the protein chain held together by disulfide bridges, hydrophobic/hydrophilic interactions, and other bonds Quaternary st ...
... Primary structure is the sequence of amino acids linked together in a protein Secondary structures are helices and pleated sheets Tertiary structure refers to complex foldings of the protein chain held together by disulfide bridges, hydrophobic/hydrophilic interactions, and other bonds Quaternary st ...
Simulating Protein Synthesis
... List at least 3 differences between transcription and translation? (3) Transcription ...
... List at least 3 differences between transcription and translation? (3) Transcription ...
Plasma membrane
... Enzymatic activity – proteins may be enzymes that catalyze steps in metabolic pathway Signal transduction – protein is a receptor for chemical messenger (hormone). Conformational change in protein relays message to inside of cell Intercellular joining – membrane proteins of adjacent cells join toget ...
... Enzymatic activity – proteins may be enzymes that catalyze steps in metabolic pathway Signal transduction – protein is a receptor for chemical messenger (hormone). Conformational change in protein relays message to inside of cell Intercellular joining – membrane proteins of adjacent cells join toget ...
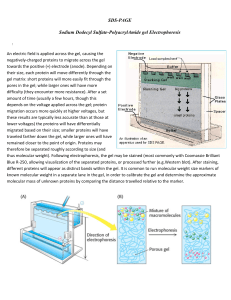
SDS-PAGE Sodium Dodecyl Sulfate
... difficulty (they encounter more resistance). After a set amount of time (usually a few hours, though this depends on the voltage applied across the gel; protein migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages) the protei ...
... difficulty (they encounter more resistance). After a set amount of time (usually a few hours, though this depends on the voltage applied across the gel; protein migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages) the protei ...
ATP
... Protein Structure • 2 or more amino acids joined by peptide bond –Hence the other name for a protein: polypeptide chain ...
... Protein Structure • 2 or more amino acids joined by peptide bond –Hence the other name for a protein: polypeptide chain ...
Isofocusing Chromatography
... the PH. The PI of each protein is the PH at which the protein has zero surface charge. •When the PH above its isoelectric point, protein will bind to positively charged medium or anion exchanger. •When the PH below its PI, protein will bind to negatively charged medium or cation exchanger. •In isofo ...
... the PH. The PI of each protein is the PH at which the protein has zero surface charge. •When the PH above its isoelectric point, protein will bind to positively charged medium or anion exchanger. •When the PH below its PI, protein will bind to negatively charged medium or cation exchanger. •In isofo ...
Night Time Muscle Growth
... muscles with extra amino acids while they sleep, and that's good for muscle growth, they say. And they're right too: Dutch nutritionists confirmed the theory this month in Medicine & Science in Sports & Exercise. The research was carried out by sports nutritionist Peter Res and is a first. It's the ...
... muscles with extra amino acids while they sleep, and that's good for muscle growth, they say. And they're right too: Dutch nutritionists confirmed the theory this month in Medicine & Science in Sports & Exercise. The research was carried out by sports nutritionist Peter Res and is a first. It's the ...
Nitrogen Balance
... • Ammonia is the major end product of N-metabolism in almost all osteichthyes • The largest source of ammonia is catabolism of dietary or structural protein • Essential amino acids in fish are the same as those in mammals: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, ...
... • Ammonia is the major end product of N-metabolism in almost all osteichthyes • The largest source of ammonia is catabolism of dietary or structural protein • Essential amino acids in fish are the same as those in mammals: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, ...
Unit 4: Cells
... Lipids can be used to store energy. Some are an important part of membranes and waterproof coverings. ...
... Lipids can be used to store energy. Some are an important part of membranes and waterproof coverings. ...
File
... that transport electrons in cellular processes. Cyclic AMP (cAMP) is critical in certain hormone function. ...
... that transport electrons in cellular processes. Cyclic AMP (cAMP) is critical in certain hormone function. ...
Amino Acids 14.5 * 14.8
... Occur in some proteins, but not all. Derived from common amino acids. Produced in a process called post-translational ...
... Occur in some proteins, but not all. Derived from common amino acids. Produced in a process called post-translational ...
A1980JQ46200001
... spectroscopy was at the Carlsberg Laboratory where I was a postdoctoral visitor with K. Linderstrøm-Lang. I applied the then rather new technique of difference spectroscopy in model compound studies to test Crammer and Neuberger’s suggestion that some of ovalbumin’s tyrosyl residues were Hbonded to ...
... spectroscopy was at the Carlsberg Laboratory where I was a postdoctoral visitor with K. Linderstrøm-Lang. I applied the then rather new technique of difference spectroscopy in model compound studies to test Crammer and Neuberger’s suggestion that some of ovalbumin’s tyrosyl residues were Hbonded to ...
biologically important molecules
... Peptide bond between amino and carboxyl. The reverse reaction is HYDROLYSIS and is performed by PROTEASE enzyme such as PEPSIN or TRYPSIN. ...
... Peptide bond between amino and carboxyl. The reverse reaction is HYDROLYSIS and is performed by PROTEASE enzyme such as PEPSIN or TRYPSIN. ...
Protein Structure
... Select the type of tertiary interaction as (1) disulfide (2) ionic (3) H bonds (4) hydrophobic A. B. C. D. ...
... Select the type of tertiary interaction as (1) disulfide (2) ionic (3) H bonds (4) hydrophobic A. B. C. D. ...
Amino Acids, Proteins, and Enzymes
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
Amino Acids, Proteins, and Enzymes
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
Laura Bassi Centres of Expertise - PlantBioP Plant
... have demonstrated that certain N-glycan residues significantly enhance therapeutic potency. Consequently glycosylation is playing an increasingly important role in drug development. The expression systems in current use, which are mainly based on mammalian cells, allow only limited control over this ...
... have demonstrated that certain N-glycan residues significantly enhance therapeutic potency. Consequently glycosylation is playing an increasingly important role in drug development. The expression systems in current use, which are mainly based on mammalian cells, allow only limited control over this ...
Supplementary File S2: analysis of protein-protein
... adhesion (50%), cell differentiation (44%) and cell migration (19%). Proteins in cluster 2 are involved in transmembrane receptor protein kinase signalling (38%) and Ephrin signalling in particular (23%), as well as cell differentiation (46%), angiogenesis (15%) and, interestingly, neurogenesis (38% ...
... adhesion (50%), cell differentiation (44%) and cell migration (19%). Proteins in cluster 2 are involved in transmembrane receptor protein kinase signalling (38%) and Ephrin signalling in particular (23%), as well as cell differentiation (46%), angiogenesis (15%) and, interestingly, neurogenesis (38% ...
Amino Acids, Proteins, and Enzymes
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
... Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet B. 1 Order of amino acids in a protein C. 4 A protein with two or more peptide chains D. 3 The shape of a globular protein E. 3 Disulfide bonds between R groups ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.