Biochem Review
... 8. They are ___________ - they can be used over, and over again because they are not destroyed and their shape does not change 9. They are ____________ - SHAPE MATTERS!! 10. What is the function of enzymes in biological systems? Why are they necessary for all biochemical reactions? 11. Explain why e ...
... 8. They are ___________ - they can be used over, and over again because they are not destroyed and their shape does not change 9. They are ____________ - SHAPE MATTERS!! 10. What is the function of enzymes in biological systems? Why are they necessary for all biochemical reactions? 11. Explain why e ...
AP BIO REVIEW ~ UNIT 1 BIOCHEMISTRY
... **You must be able to recognize the structures of these compounds – study the pictures! 1. CARBOHYDRATES Used by the cells of the body - in energy-producing reactions - as structural materials Classified into 3 groups according to the number of sugar (saccharide) molecules: 1) Monsaccharide: “si ...
... **You must be able to recognize the structures of these compounds – study the pictures! 1. CARBOHYDRATES Used by the cells of the body - in energy-producing reactions - as structural materials Classified into 3 groups according to the number of sugar (saccharide) molecules: 1) Monsaccharide: “si ...
Solutions to 7.012 Problem Set 1
... cellulose. You want to design an enzyme that will bind this small polypeptide. You design the enzyme shown below. b) In the table below please indicate which amino acid you placed in each position to give the strongest possible interaction between your enzyme and the small polypeptide. For instance, ...
... cellulose. You want to design an enzyme that will bind this small polypeptide. You design the enzyme shown below. b) In the table below please indicate which amino acid you placed in each position to give the strongest possible interaction between your enzyme and the small polypeptide. For instance, ...
2.4 Chemical Reactions and Enzymes
... build up in the body faster than the bloodstream could remove it. Our bloodstream contains an enzyme called carbonic anhydrase that speeds up the reaction by a factor of 10 million. The reaction takes place immediately & CO2 is removed from the blood instantly. ...
... build up in the body faster than the bloodstream could remove it. Our bloodstream contains an enzyme called carbonic anhydrase that speeds up the reaction by a factor of 10 million. The reaction takes place immediately & CO2 is removed from the blood instantly. ...
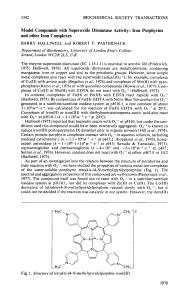
Model Compounds with Superoxide Dismutase Activity: Iron
... (Halliwell, 1975). By competition of Fe(I1)-EDTA with Nitro Blue Tetrazolium for 02-* generated in a xanthine/xanthine oxidase system at pH 10.1, a rate constant of about 3 x 105~-'*s-' was calculated for the reaction of Fe(l1)-EDTA with 02-'at 20°C. Complexes of iron(I1) or iron(II1) with diethylen ...
... (Halliwell, 1975). By competition of Fe(I1)-EDTA with Nitro Blue Tetrazolium for 02-* generated in a xanthine/xanthine oxidase system at pH 10.1, a rate constant of about 3 x 105~-'*s-' was calculated for the reaction of Fe(l1)-EDTA with 02-'at 20°C. Complexes of iron(I1) or iron(II1) with diethylen ...
BICH 605
... Binding is pH dependent and elution is carried out by reducing the pH or increasing ionic strength of the buffer. Another widely used elution method is to use EDTA in the buffer. This method is very useful and widely used to purify recombinant proteins that have been made as over-expressed, Histag f ...
... Binding is pH dependent and elution is carried out by reducing the pH or increasing ionic strength of the buffer. Another widely used elution method is to use EDTA in the buffer. This method is very useful and widely used to purify recombinant proteins that have been made as over-expressed, Histag f ...
Chapter 3
... • Single polynucleotide strand • RNA uses information in DNA to specify sequence of amino acids in proteins ...
... • Single polynucleotide strand • RNA uses information in DNA to specify sequence of amino acids in proteins ...
Atomic Nature of Matter
... • Chart that lists atoms by their atomic number and by their electron arrangements. • Elements arranged so chemical and physical properties have trends following a pattern of ...
... • Chart that lists atoms by their atomic number and by their electron arrangements. • Elements arranged so chemical and physical properties have trends following a pattern of ...
Exam 2
... Bile salts chylomicrons All of the above are involved in lipid digestion, transport, and storage. ...
... Bile salts chylomicrons All of the above are involved in lipid digestion, transport, and storage. ...
Chapter 21 - Cengage Learning
... answers to these questions lie in the biochemistry of the human body. To begin to understand how the complex biochemical systems work, we need to know about the kinds of molecules that are important in living organisms. They are often large organic molecules, and they contain atoms and functional gr ...
... answers to these questions lie in the biochemistry of the human body. To begin to understand how the complex biochemical systems work, we need to know about the kinds of molecules that are important in living organisms. They are often large organic molecules, and they contain atoms and functional gr ...
UBC Dairy Education and Research Centre
... 80% of the atmosphere composed of N2 This N is unavailable for plant nutrition Ammonia (NH3 ) is the only form of nitrogen that can be utilized by the plant ...
... 80% of the atmosphere composed of N2 This N is unavailable for plant nutrition Ammonia (NH3 ) is the only form of nitrogen that can be utilized by the plant ...
Organic Chemistry - Welcome to Cherokee High School
... Organic Compounds • Organic Compounds often form Polymers • Long chains of smaller molecules (not atoms) called monomers, bind to form huge Macromolecules • 4 Types: Carbohydrates, Lipids, Proteins & ...
... Organic Compounds • Organic Compounds often form Polymers • Long chains of smaller molecules (not atoms) called monomers, bind to form huge Macromolecules • 4 Types: Carbohydrates, Lipids, Proteins & ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 15. Which dn configurations show quenching of orbital angular momentum if it forms octahedral, high and low spin complexes? Give reasons. 16. Discuss the synthesis and uses of cis-platin. 17. What is Marcus Hush equation? What is its application? 18. Write a brief notes on Fischer Tropsch process. 1 ...
... 15. Which dn configurations show quenching of orbital angular momentum if it forms octahedral, high and low spin complexes? Give reasons. 16. Discuss the synthesis and uses of cis-platin. 17. What is Marcus Hush equation? What is its application? 18. Write a brief notes on Fischer Tropsch process. 1 ...
Reading Guide
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
CP Chemistry Worksheet: Ions
... 1. For each of the positive ions listed in column 1, use the periodic table to find in column 2 the total number of electrons that ion contains. The same answer may be used more than once. _B__ 1. Al+3 A. 2 _D__ 2. Fe+3 ...
... 1. For each of the positive ions listed in column 1, use the periodic table to find in column 2 the total number of electrons that ion contains. The same answer may be used more than once. _B__ 1. Al+3 A. 2 _D__ 2. Fe+3 ...
CP Chemistry Worksheet: Ions
... 4. Write the chemical symbol for the ion with 12 protons and 10 electrons. 5. Write the chemical symbol for the ion with 74 protons and 68 electrons. 6. Write the chemical symbol for the ion with 95 protons and 89 electrons. 7. Write the chemical symbol for the ion with 33 protons and 36 electrons. ...
... 4. Write the chemical symbol for the ion with 12 protons and 10 electrons. 5. Write the chemical symbol for the ion with 74 protons and 68 electrons. 6. Write the chemical symbol for the ion with 95 protons and 89 electrons. 7. Write the chemical symbol for the ion with 33 protons and 36 electrons. ...
Soluble salts
... NaCl(s) + H2O(l) ---> NaCl(aq) How sodium chloride exists in solution. ____________________________________________ ________ electrolyte are solutes that exist in solution predominantly in the form of hydrated (solvated) molecules. A good example is oxalic acid dissolved in water. H2C2O4(s) + H2O(l) ...
... NaCl(s) + H2O(l) ---> NaCl(aq) How sodium chloride exists in solution. ____________________________________________ ________ electrolyte are solutes that exist in solution predominantly in the form of hydrated (solvated) molecules. A good example is oxalic acid dissolved in water. H2C2O4(s) + H2O(l) ...
Using mass to calculate molecular formula
... Empirical formula and Molecular formula Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number o ...
... Empirical formula and Molecular formula Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number o ...
Worksheet 20.2
... 1- Atoms can achieve a noble gas structure by gaining, losing or sharing electrons with other atoms. 2- The rule states that, except for hydrogen , an atom combines with other atoms to form bonds in order to have 8 electrons in its valence energy level ( like noble gases). Lewis dot symbols are repr ...
... 1- Atoms can achieve a noble gas structure by gaining, losing or sharing electrons with other atoms. 2- The rule states that, except for hydrogen , an atom combines with other atoms to form bonds in order to have 8 electrons in its valence energy level ( like noble gases). Lewis dot symbols are repr ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.