* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Worksheet 20.2
Coordination complex wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Atomic orbital wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bent's rule wikipedia , lookup
Hydrogen bond wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Halogen bond wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electronegativity wikipedia , lookup
Metalloprotein wikipedia , lookup
Bond valence method wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electron configuration wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic theory wikipedia , lookup
Metallic bonding wikipedia , lookup
History of molecular theory wikipedia , lookup
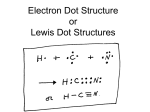
Grade 9 Physical Science Student Name: Worksheet for Section 20.2: Chemical Bonding. General Information: 1- Atoms can achieve a noble gas structure by gaining, losing or sharing electrons with other atoms. 2- The rule states that, except for hydrogen , an atom combines with other atoms to form bonds in order to have 8 electrons in its valence energy level ( like noble gases). Lewis dot symbols are representations of the elements which give a dot ( . ) for each valence electron on the atom. The Lewis dot symbols for the period 2 elements are: Be Li B C N O F Ne 1. Fill in the Lewis dot symbols for: Ga P Br Ca Si Lewis dot structures allow us to understand two types of bonding, Ionic and Covalent. Ionic Bonds Ionic bonds are usually formed by the reaction of metals with non-metals. Example: Fill in the Lewis dot symbols for Na and Cl, below, and complete the shorthand electron configuration for each: Na Cl Now allow each atom to complete its Valence energy level with 8 electrons, by losing or gaining electrons. Then write the Lewis structure for each ion and its charge. Na Cl Sodium loses an e-because it’s a metal Chlorine gains an e- because it’s non-metals. 2. 4. Use Lewis dot symbols to show the transfer of electrons between the following atoms to form ions: a) K and S b) O and Ba c) Ca and F d) Al and Cl e) Mg and O Covalent Bonds These are bonds formed between non-metals. So octets of valence electrons are obtained by sharing electrons, instead of gaining or losing electrons. An example of covalent bonding is F2, fluorine gas. 3. Complete the Lewis dot symbols for the fluorine atoms below F F 4. Use the Lewis diagram/structure to show how H and O react to form water, H2O. 5. Draw the Lewis diagram/structure to show how N and H react to form Ammonia, NH3. 6. Draw the Lewis structure of Nitrogen, N2. 7. Draw the Lewis structure of Oxygen, O2. 8. Draw the Lewis structure of Hydrogen, H2. 9. Draw the Lewis structure of Hydrogen chloride, HCl 10. Label (ionic) or (covalent) the bonds in the following compounds. CO2 Na2O SF2 N2O CaO Na2CO3 (careful) 11. Fill the following table to compare between Ionic and Covalent bond Criteria How does it form Ionic Bond Electron transfer Covalent bond Type of reacted elements Nonmetal with non metal Name of resulting compound Molecule 12. Which is more stable Cl or Cl- Ion? Why? 13. Assign the following as: Ionic, Polar covalent or nonpolar covalent : a) C-Cl b)N-F c) C-F d) H-Cl e) Mg-O f) C-O g) O-O h) Si-O i) N-O