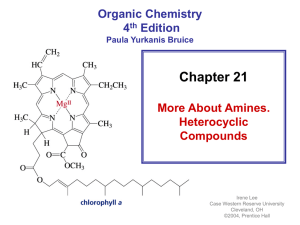
BI ACE_02 .
... In solution, amino acids will ionize The amino group and carboxyl group will do so. The carboxyl group produces Hydrogen ions and acts like an acid, while the amino group removes Hydrogen ions from solution, acting as a base. ...
... In solution, amino acids will ionize The amino group and carboxyl group will do so. The carboxyl group produces Hydrogen ions and acts like an acid, while the amino group removes Hydrogen ions from solution, acting as a base. ...
Chemical Formula Notes
... Coefficient the number placed in front of a chemical formula to represent the number of molecules of a certain substance. ...
... Coefficient the number placed in front of a chemical formula to represent the number of molecules of a certain substance. ...
Proteins - Many Structures, Many Functions
... • A protein’s specific conformation determines its function. • In almost every case, the function depends on its ability to recognize and bind to some other molecule. – For example, antibodies bind to particular foreign substances that fit their binding sites. – Enzyme recognize and bind to specifi ...
... • A protein’s specific conformation determines its function. • In almost every case, the function depends on its ability to recognize and bind to some other molecule. – For example, antibodies bind to particular foreign substances that fit their binding sites. – Enzyme recognize and bind to specifi ...
ST110 Chemistry, Cellular Structure, and Function_BB
... – Weak in comparison to ionic or covalent bonds – Positive hydrogen atoms attract negative molecules – Hydrogen bonds hold water molecules together in the liquid form ...
... – Weak in comparison to ionic or covalent bonds – Positive hydrogen atoms attract negative molecules – Hydrogen bonds hold water molecules together in the liquid form ...
Reagents for Protein Sequence DeterminaXon
... peptides and reduction of peptide by one residue 3. N-terminal analysis: for reaction with amino terminus of proteins/ peptides, but complete hydrolysis of peptide required a. FDNB b. Dansyl chloride c. Fluorescamine d. o-phthalaldehyde ...
... peptides and reduction of peptide by one residue 3. N-terminal analysis: for reaction with amino terminus of proteins/ peptides, but complete hydrolysis of peptide required a. FDNB b. Dansyl chloride c. Fluorescamine d. o-phthalaldehyde ...
Document
... Weak bonds also occur throughout the polypeptide, between the amino acid side chains in regions of secondary structure as well as the looped regions ...
... Weak bonds also occur throughout the polypeptide, between the amino acid side chains in regions of secondary structure as well as the looped regions ...
No Slide Title
... In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes colo ...
... In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes colo ...
Chapter 4 - Reactions in Aqueous Solutions
... In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes colo ...
... In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes colo ...
Example of the Course Test 2 10th December, 8:00, registration from
... a) reaction: CH3-CO-COOH + NAD+ + HSCoA -> CO2 + NADH + H+ + CH3-CO~SCoA describes a decarboxylation of oxaloacetate b) glucose can be metabolised to lactate in erythrocytes c) insulin activates only anabolic pathways d) adenylate kinase catalyzes this reaction: ADP + ADP = AMP + ATP 2) Choose true ...
... a) reaction: CH3-CO-COOH + NAD+ + HSCoA -> CO2 + NADH + H+ + CH3-CO~SCoA describes a decarboxylation of oxaloacetate b) glucose can be metabolised to lactate in erythrocytes c) insulin activates only anabolic pathways d) adenylate kinase catalyzes this reaction: ADP + ADP = AMP + ATP 2) Choose true ...
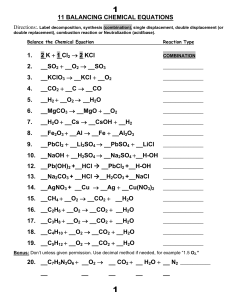
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... 10. In “2s2 “, the 2 represents which 3 Æ (column, group, family, period, row or energy level). 7 Ions and Bonding 11. Which 2 have 3 valence electron? ...
... 10. In “2s2 “, the 2 represents which 3 Æ (column, group, family, period, row or energy level). 7 Ions and Bonding 11. Which 2 have 3 valence electron? ...
Amino Acid Biosynthesis Student Companion Ch 24 Self Test
... 5) Alpha-ketoglutarate provides the carbon skeleton for which amino acids? 6) Two different amidation methods are used to install side chain amides in amino acids. Describe these two methods and match them to the relevant amino acid. 7) Which amino acids derive their carbon skeletons completely from ...
... 5) Alpha-ketoglutarate provides the carbon skeleton for which amino acids? 6) Two different amidation methods are used to install side chain amides in amino acids. Describe these two methods and match them to the relevant amino acid. 7) Which amino acids derive their carbon skeletons completely from ...
Slide notes
... But as more base is added, the white preciptate dissolves, forming the complex ion Zn(OH)42-. Other common amphoteric hydroxides are those of aluminum, chromium(III), lead(II), tin(II), and tin(IV). ...
... But as more base is added, the white preciptate dissolves, forming the complex ion Zn(OH)42-. Other common amphoteric hydroxides are those of aluminum, chromium(III), lead(II), tin(II), and tin(IV). ...
Exam #3 2 Problem 1. (25 points) You study ligand binding to two
... Carbon monoxide, an odorless gas, binds to hemoglobin to form CO-hemoglobin. Crystals of CO-hemoglobin are isomorphous with those of oxyhemoglobin, which suggests that COHb has the same conformation as oxyhemoglobin. Each heme in Hb can bind one CO molecule, but O2 and CO cannot simultaneously bind ...
... Carbon monoxide, an odorless gas, binds to hemoglobin to form CO-hemoglobin. Crystals of CO-hemoglobin are isomorphous with those of oxyhemoglobin, which suggests that COHb has the same conformation as oxyhemoglobin. Each heme in Hb can bind one CO molecule, but O2 and CO cannot simultaneously bind ...
Organic Chemistry – Review #2 Vocabulary Adhesion Cohesion
... Catalysts increase the rate of a reaction without being changed by the reaction, catalysts lower the activation required for the reaction to proceed. Substrates are the reactants on which enzymes (catalysts) work Rate of reaction in both directions is increased by the presence of specific enzymes. _ ...
... Catalysts increase the rate of a reaction without being changed by the reaction, catalysts lower the activation required for the reaction to proceed. Substrates are the reactants on which enzymes (catalysts) work Rate of reaction in both directions is increased by the presence of specific enzymes. _ ...
Protein Engineering
... • Consist of linear polymers built from series of up to 20 different Lamino acids • Play central roles in biological events through interactions with cognate proteins / ligands - Cell signaling processes • Enzymes catalyze the reactions with high specificity and catalytic efficiency in cellular meta ...
... • Consist of linear polymers built from series of up to 20 different Lamino acids • Play central roles in biological events through interactions with cognate proteins / ligands - Cell signaling processes • Enzymes catalyze the reactions with high specificity and catalytic efficiency in cellular meta ...
matter crct/final exam review
... 81. Describe the energy content of the reactants versus the energy content of the products in both an endothermic and exothermic reaction. ...
... 81. Describe the energy content of the reactants versus the energy content of the products in both an endothermic and exothermic reaction. ...
Theory
... The strength of carboxylic acid is, again, dependent of inductive effect. In case of carboxylic acid with long aliphatic chain, polarity of O-H bond in carboxylic group is lowered (electrons are moved from this chain towards COOH – positive inductive effect) and proton is not so “keen” to be dissoc ...
... The strength of carboxylic acid is, again, dependent of inductive effect. In case of carboxylic acid with long aliphatic chain, polarity of O-H bond in carboxylic group is lowered (electrons are moved from this chain towards COOH – positive inductive effect) and proton is not so “keen” to be dissoc ...
American-Journal-of-Oil-and-Chemical-Technologies
... In conclusion, continuing with our previous works on synthesizing supramolecular compounds containing pyridinedicarboxylic acid N-oxides [9-11], two new coordination complexes have been synthesized and characterized. The red shift of bands ʋas(COO−), ʋs(COO−), and ʋas(COO−) and ʋ(NO) confirm formati ...
... In conclusion, continuing with our previous works on synthesizing supramolecular compounds containing pyridinedicarboxylic acid N-oxides [9-11], two new coordination complexes have been synthesized and characterized. The red shift of bands ʋas(COO−), ʋs(COO−), and ʋas(COO−) and ʋ(NO) confirm formati ...
Chapter 2
... carbon and hydrogen Inorganic compounds typically lack carbon Water Inorganic Polar molecule Solvent Polar substances dissociate,forming solutes H+ and OH participate in chemical reactions H bonds absorb heat Makes water a temperature buffer ...
... carbon and hydrogen Inorganic compounds typically lack carbon Water Inorganic Polar molecule Solvent Polar substances dissociate,forming solutes H+ and OH participate in chemical reactions H bonds absorb heat Makes water a temperature buffer ...
N -glutamate Iminohydrolase from Pseudomonas aeruginosa L
... subunit and a specific activity of 5.2 s-1. Incubation of HutF with the metal chelator dipicolinate for 3 h at pH 5.6 was effective in the removal of zinc from the active site. A metal analysis of the apoenzyme found 0.03 equiv of zinc per subunit and a specific activity of less than 0.1 s-1. More t ...
... subunit and a specific activity of 5.2 s-1. Incubation of HutF with the metal chelator dipicolinate for 3 h at pH 5.6 was effective in the removal of zinc from the active site. A metal analysis of the apoenzyme found 0.03 equiv of zinc per subunit and a specific activity of less than 0.1 s-1. More t ...
Chapter 2 Notes
... example: water (H2O) 3. chemical properties = describe how one substance changes when it reacts with other substances; example: iron changes to rust when it reacts to water and oxygen ***may indicate a chemical reaction: a. color change b. gas produced c. temperature change 4. Ions- electrically cha ...
... example: water (H2O) 3. chemical properties = describe how one substance changes when it reacts with other substances; example: iron changes to rust when it reacts to water and oxygen ***may indicate a chemical reaction: a. color change b. gas produced c. temperature change 4. Ions- electrically cha ...
File
... The body cells will use only the amount of amino acids necessary to meet their protein needs. They cannot store excess amino acids. Because the human body does not have a mechanism to store excess nitrogen, it cannot store amino acids. Through the process of deamination , the amino group NH2 contain ...
... The body cells will use only the amount of amino acids necessary to meet their protein needs. They cannot store excess amino acids. Because the human body does not have a mechanism to store excess nitrogen, it cannot store amino acids. Through the process of deamination , the amino group NH2 contain ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.