Characteristics of Living Things (Essay
... Part a. What are all organic molecules are comprised of, and where are organic molecules found? What elements? Most of the atoms within organic molecules are bound together with covalent bonds...what is a covalent bond? Why does such a bond form? Part b. Explain the four main categories of organic m ...
... Part a. What are all organic molecules are comprised of, and where are organic molecules found? What elements? Most of the atoms within organic molecules are bound together with covalent bonds...what is a covalent bond? Why does such a bond form? Part b. Explain the four main categories of organic m ...
File - Mr Weng`s IB Chemistry
... • Lipids act as structural components of cell membranes, in energy storage, thermal and electrical insulation, as transporters of lipid soluble vitamins and as hormones. • Deduction of the structural formulas of reactants and products in condensation and hydrolysis reactions between glycerol and fat ...
... • Lipids act as structural components of cell membranes, in energy storage, thermal and electrical insulation, as transporters of lipid soluble vitamins and as hormones. • Deduction of the structural formulas of reactants and products in condensation and hydrolysis reactions between glycerol and fat ...
Protein And Amino Acids - Manasquan Public Schools
... Proteins function as carriers for: Vitamins Minerals Lipids Oxygen ...
... Proteins function as carriers for: Vitamins Minerals Lipids Oxygen ...
Document
... • This is cooled and left in moulds to set and then more bacteria is added to give different flavours ...
... • This is cooled and left in moulds to set and then more bacteria is added to give different flavours ...
AND BINUCLEAR COMPLEXES CONTAINING OXIME LIGANDS
... compounds containing three oximate anions as bridging ligand, where the central divalent metal ion is redox inactive. The use of oxime ligands can lead to a propeller-shaped structure in which two metal ions have deformed O2N4 coordination octahedral polyhedra, while the remaining ones are in a stro ...
... compounds containing three oximate anions as bridging ligand, where the central divalent metal ion is redox inactive. The use of oxime ligands can lead to a propeller-shaped structure in which two metal ions have deformed O2N4 coordination octahedral polyhedra, while the remaining ones are in a stro ...
Topic 3 – The Chemistry of Life
... bring substrates close together in active site / in correct orientation forms enzyme-substrate complex / substrate(s) bind to active site lowers the activation energy for the reaction ...
... bring substrates close together in active site / in correct orientation forms enzyme-substrate complex / substrate(s) bind to active site lowers the activation energy for the reaction ...
Name
... 3. Which of the following is not true about the ribosome binding site (rbs): a. inhibitory proteins can bind to the rbs and prevent translation b. the rbs is a consensus sequence c. the rbs is found on the 5’ untranslated region (UTR) d. the rbs binds to a complementary region within the small ribos ...
... 3. Which of the following is not true about the ribosome binding site (rbs): a. inhibitory proteins can bind to the rbs and prevent translation b. the rbs is a consensus sequence c. the rbs is found on the 5’ untranslated region (UTR) d. the rbs binds to a complementary region within the small ribos ...
1 1) What kinds of molecules pass through a cell membrane most
... A) amino acids and proteins. B) glycerol and fatty acids. C) glucose and sucrose. D) starch and glycogen. E) all of the above 27) A plant cell placed in a hypotonic solution will not lyse because A) the cell wall prevents the plant cell from bursting. B) plant plasma membranes are impermeable to wat ...
... A) amino acids and proteins. B) glycerol and fatty acids. C) glucose and sucrose. D) starch and glycogen. E) all of the above 27) A plant cell placed in a hypotonic solution will not lyse because A) the cell wall prevents the plant cell from bursting. B) plant plasma membranes are impermeable to wat ...
Systemic Response to Injury and Metabolic Support
... Sig amounts of protein must be degraded to be used for gluconeogenesis Urine nitrogen excretion increases from 7-10g/day to up to 30g/day Protein degradation occurs mostly in skeletal muscles, but also some in solid organs ...
... Sig amounts of protein must be degraded to be used for gluconeogenesis Urine nitrogen excretion increases from 7-10g/day to up to 30g/day Protein degradation occurs mostly in skeletal muscles, but also some in solid organs ...
An introduction to the virtual issue on Coordination
... compounds is that they all consist of metal-containing nodes that are ‘infinitely’ bridged by exo-multidentate ligands. Unfortunately there has been little consistency in the literature with regard to the terminology used to describe such materials and it is therefore essential to preface any credib ...
... compounds is that they all consist of metal-containing nodes that are ‘infinitely’ bridged by exo-multidentate ligands. Unfortunately there has been little consistency in the literature with regard to the terminology used to describe such materials and it is therefore essential to preface any credib ...
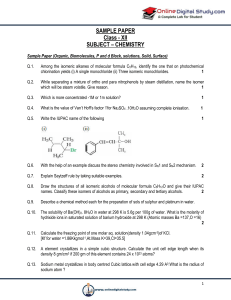
SAMPLE PAPER Class - XII SUBJECT
... Ferric hydroxide sol gets coagulated on addition of sodium chloride solution (b) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories. (c) Physical adsorption is multilayered, while chemisorption is monolayered. Q.17. Nitro group increases the reactivity of chloroben ...
... Ferric hydroxide sol gets coagulated on addition of sodium chloride solution (b) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories. (c) Physical adsorption is multilayered, while chemisorption is monolayered. Q.17. Nitro group increases the reactivity of chloroben ...
Review Problems #2 (Enzyme Review, Phosphatases
... 2) The branchpoint for aromatic amino acid biosynthesis is chorismate. What is the structure of chorismate? What are the three immediate products derived from chorismate that constitute the first unique steps in the synthesis of the three aromatic amino acids? 3) From where are the two carbons of th ...
... 2) The branchpoint for aromatic amino acid biosynthesis is chorismate. What is the structure of chorismate? What are the three immediate products derived from chorismate that constitute the first unique steps in the synthesis of the three aromatic amino acids? 3) From where are the two carbons of th ...
The lithosphere (silicon dioxide)
... This is because of the strong covalent bonds It is an electrical insulator ...
... This is because of the strong covalent bonds It is an electrical insulator ...
The Lithosphere – Silicon Dioxide
... This is because of the strong covalent bonds It is an electrical insulator ...
... This is because of the strong covalent bonds It is an electrical insulator ...
5.4 Molecular Models for Plants Growing: Biosynthesis PPT
... from glucose and minerals The result of photosynthesis is glucose, then plants use the glucose to make other small organic molecules (monomers). ...
... from glucose and minerals The result of photosynthesis is glucose, then plants use the glucose to make other small organic molecules (monomers). ...
BiochemLecture03
... acids with respect to the conforomations that the backbone can adopt. For this reason, it is not surprising to see Alanine present in just about all non-critical protein contexts. • Role in function: The Alanine side chain is very nonreactive, and is thus rarely directly involved in protein function ...
... acids with respect to the conforomations that the backbone can adopt. For this reason, it is not surprising to see Alanine present in just about all non-critical protein contexts. • Role in function: The Alanine side chain is very nonreactive, and is thus rarely directly involved in protein function ...
Substrate-Promoted Formation of a Catalytically Competent
... i.e., water/hydroxide ligands, coordinated to one or both metal ions. While it is at present not possible to unambiguously distinguish between these two possibilities, a reaction mechanism is proposed whereby the terminally bound H2O/OH- acts as the nucleophile, activated via hydrogen bonding by the ...
... i.e., water/hydroxide ligands, coordinated to one or both metal ions. While it is at present not possible to unambiguously distinguish between these two possibilities, a reaction mechanism is proposed whereby the terminally bound H2O/OH- acts as the nucleophile, activated via hydrogen bonding by the ...
Biochemistry Review Reteach
... 24. A fatty acid containing at least two double bonds is called (a.) cholesterol (b.) saturated (c.) polyunsaturated (d.) dehydrogenase (e.) monounsaturated 25. Which is NOT a function of carbohydrates (as a class)? (a.) Structural support (b.) Immediate energy (c.) Energy storage (d.) Enzymatic cat ...
... 24. A fatty acid containing at least two double bonds is called (a.) cholesterol (b.) saturated (c.) polyunsaturated (d.) dehydrogenase (e.) monounsaturated 25. Which is NOT a function of carbohydrates (as a class)? (a.) Structural support (b.) Immediate energy (c.) Energy storage (d.) Enzymatic cat ...
Balancing ANY chemical Equation
... • Electrolytes: Substances that form ions when dissolved in solution. Electrolytes can be weak or strong. • Strong Electrolytes: Substances that completely separate into their component ions when dissolved. (All soluble ionic compounds and strong acids are strong electrolytes.) • Weak Electrolytes: ...
... • Electrolytes: Substances that form ions when dissolved in solution. Electrolytes can be weak or strong. • Strong Electrolytes: Substances that completely separate into their component ions when dissolved. (All soluble ionic compounds and strong acids are strong electrolytes.) • Weak Electrolytes: ...
Chem Bonding Notes
... (1) A chemical bond is broken and energy is released. (2) A chemical bond is broken and energy is absorbed. (3) A chemical bond is formed and energy is released. (4) A chemical bond is formed and energy is absorbed. 5. Which molecule is nonpolar? (1) H20 (3)CO (2) NH3 (4) C02 6. What is the correct ...
... (1) A chemical bond is broken and energy is released. (2) A chemical bond is broken and energy is absorbed. (3) A chemical bond is formed and energy is released. (4) A chemical bond is formed and energy is absorbed. 5. Which molecule is nonpolar? (1) H20 (3)CO (2) NH3 (4) C02 6. What is the correct ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.