lecture 3
... with things other than water Fact: - association constants (ka) increase significantly - dissociation constants (kd) decrease significantly (kd=1/ka) - increased on-rates for protein-protein interactions (see for example Rohwer et al. (2000) J. Biol. Chem. 275, 34909) ...
... with things other than water Fact: - association constants (ka) increase significantly - dissociation constants (kd) decrease significantly (kd=1/ka) - increased on-rates for protein-protein interactions (see for example Rohwer et al. (2000) J. Biol. Chem. 275, 34909) ...
The SPFH domain - Tavernarakis Lab
... of the L-selectin is quenched by putative cis-interactions32 with oligosaccharide ligands on the same cell surface. The L-selectin on leukocyte 3 is shed from the cell surface. Endothelial cells II, III and V are able to support L-selectin-mediated leukocyte adhesion on account of their having at th ...
... of the L-selectin is quenched by putative cis-interactions32 with oligosaccharide ligands on the same cell surface. The L-selectin on leukocyte 3 is shed from the cell surface. Endothelial cells II, III and V are able to support L-selectin-mediated leukocyte adhesion on account of their having at th ...
Protein
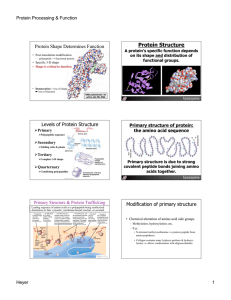
... tRNA’s line up one after the other with amino acids Amino acids form peptide bonds to make the primary sequence of the protein Protein then coils to form the secondary and tertiary structure ...
... tRNA’s line up one after the other with amino acids Amino acids form peptide bonds to make the primary sequence of the protein Protein then coils to form the secondary and tertiary structure ...
Powerpoint Presentation: Proteins
... This folding is sometimes held together by strong covalent bonds (e.g. cysteine-cysteine disulphide bridge) Bending of the chain takes place at certain amino acids (e.g. proline) Hydrophobic amino acids tend to arrange themselves inside the molecule Hydrophilic amino acids arrange themselves on the ...
... This folding is sometimes held together by strong covalent bonds (e.g. cysteine-cysteine disulphide bridge) Bending of the chain takes place at certain amino acids (e.g. proline) Hydrophobic amino acids tend to arrange themselves inside the molecule Hydrophilic amino acids arrange themselves on the ...
SouthernHybridization - University of Hawaii
... • Detect PDI protein in wild type plants. • In mutant plants, determine the effect of the T-DNA insert on the expression of the PDI gene through movement or deletion of PDI protein band. ...
... • Detect PDI protein in wild type plants. • In mutant plants, determine the effect of the T-DNA insert on the expression of the PDI gene through movement or deletion of PDI protein band. ...
Proteins File
... This folding is sometimes held together by strong covalent bonds (e.g. cysteine-cysteine disulphide bridge) Bending of the chain takes place at certain amino acids (e.g. proline) Hydrophobic amino acids tend to arrange themselves inside the molecule Hydrophilic amino acids arrange themselves on the ...
... This folding is sometimes held together by strong covalent bonds (e.g. cysteine-cysteine disulphide bridge) Bending of the chain takes place at certain amino acids (e.g. proline) Hydrophobic amino acids tend to arrange themselves inside the molecule Hydrophilic amino acids arrange themselves on the ...
Proteins
... water and unaffected by moderate changes in temperature and pH. Subgroups within this category include: Collagens & Elastins, the proteins of connective tissues. tendons and ligaments. Keratins, proteins that are major components of skin, hair, feathers and horn. Fibrin, a protein formed when blood ...
... water and unaffected by moderate changes in temperature and pH. Subgroups within this category include: Collagens & Elastins, the proteins of connective tissues. tendons and ligaments. Keratins, proteins that are major components of skin, hair, feathers and horn. Fibrin, a protein formed when blood ...
C h e m g u id e –... PROTEINS: STRUCTURE
... This diagram (also modified from the Chemguide page) shows some of the bits of the protein chain in the spirals. d) What name is given to the spirals? e) Name the intermolecular forces holding the spiral together and mark them on the diagram. f) What is represented by the string-like sections of the ...
... This diagram (also modified from the Chemguide page) shows some of the bits of the protein chain in the spirals. d) What name is given to the spirals? e) Name the intermolecular forces holding the spiral together and mark them on the diagram. f) What is represented by the string-like sections of the ...
realburn
... The kinetics of protein denaturation is affected by so many different factors: density, solvent, bond strengths, interactions with surrounding molecules. ...
... The kinetics of protein denaturation is affected by so many different factors: density, solvent, bond strengths, interactions with surrounding molecules. ...
THE PUZZLING PROPERTIES OF THE PERMEASE (PPP) Kim …
... However, stronger evidence would be obtained by comparing each hydrophilic region in a pairwise fashion. TMAP allowed us to obtain a more precise prediction of which segments were hydrophobic vs. hydrophilic. There were 9 predicted transmembrane regions. Finally, each hydrophilic segment was submit ...
... However, stronger evidence would be obtained by comparing each hydrophilic region in a pairwise fashion. TMAP allowed us to obtain a more precise prediction of which segments were hydrophobic vs. hydrophilic. There were 9 predicted transmembrane regions. Finally, each hydrophilic segment was submit ...
Aalborg Universitet Christiansen, Gunna; Sennels, Lau; Stensballe, Allan; Birkelund, Svend
... The coding capacity of the chlamydial genome was revealed by genome sequencing of strain D/UW-Cx (Stephens et al. 1998). Of the 894 likely protein-coding genes 255 (28%) were not similar to any known proteins indicating the uniqueness of the genus Chlamydia. Since then multiple chlamydial and parach ...
... The coding capacity of the chlamydial genome was revealed by genome sequencing of strain D/UW-Cx (Stephens et al. 1998). Of the 894 likely protein-coding genes 255 (28%) were not similar to any known proteins indicating the uniqueness of the genus Chlamydia. Since then multiple chlamydial and parach ...
1.0 amino acids as units of protein structure
... Cells contain thousands of different proteins. A major problem for protein chemists is to purify a chosen protein so that they can study its specific properties in the absence of other proteins. Because the biological function of a protein depends on its native structure, techniques employed in prot ...
... Cells contain thousands of different proteins. A major problem for protein chemists is to purify a chosen protein so that they can study its specific properties in the absence of other proteins. Because the biological function of a protein depends on its native structure, techniques employed in prot ...
View PDF
... number of bases and their position in the original sequence is not allowed by nature. There is no mistake possible and if it does, the result is a pathological disease [1-2]. To prevent any error in the protein translation process from gene and RNA, eukaryotic (and prokaryotic) cells have developed ...
... number of bases and their position in the original sequence is not allowed by nature. There is no mistake possible and if it does, the result is a pathological disease [1-2]. To prevent any error in the protein translation process from gene and RNA, eukaryotic (and prokaryotic) cells have developed ...
Make notes using these questions
... • Regions of hydrophobic R groups allow strong hydrophobic interactions that hold integral proteins, those embedded in the membrane, within the phospholipid bilayer as they are free to interact with the hydrophobic tails of the phospholipids. • Some integral proteins are transmembrane and cross the ...
... • Regions of hydrophobic R groups allow strong hydrophobic interactions that hold integral proteins, those embedded in the membrane, within the phospholipid bilayer as they are free to interact with the hydrophobic tails of the phospholipids. • Some integral proteins are transmembrane and cross the ...
Protein Supplements
... Pre-workout Pre-workout – designed to help the individual gain focus and increase energy and endurance for their workout. Mixtures of caffeine, creatine, select amino acids, and carbs Aids focus Increases energy ...
... Pre-workout Pre-workout – designed to help the individual gain focus and increase energy and endurance for their workout. Mixtures of caffeine, creatine, select amino acids, and carbs Aids focus Increases energy ...
Protein structure - Primary
... • This refers to the 3 dimensional folding of the chain. This structure can be globular or fibrous. The shapes give certain properties to the protein • Globular : In these the protein chain is rolled up like a ball of wool. This structure makes the protein soluble. This type of protein is found in b ...
... • This refers to the 3 dimensional folding of the chain. This structure can be globular or fibrous. The shapes give certain properties to the protein • Globular : In these the protein chain is rolled up like a ball of wool. This structure makes the protein soluble. This type of protein is found in b ...
IDENTIFICATION OF A BACTERIO
... Protein synthesis of bacteria-opsin and some other membrane proteins in vivo is selectively disturbed when Mg2+are removed from the medium, whereas no effect on the synthesis of cytoplasmic proteins can be observed. Re-addition of Mg2+to the cell suspension reconstitutes an almost normal membrane pr ...
... Protein synthesis of bacteria-opsin and some other membrane proteins in vivo is selectively disturbed when Mg2+are removed from the medium, whereas no effect on the synthesis of cytoplasmic proteins can be observed. Re-addition of Mg2+to the cell suspension reconstitutes an almost normal membrane pr ...
Folding of Proteins - Simulation using Monte Carlo
... The control in Fig. 7a refers to protein without any cross-linking. It is observed from the FTIR studies that protein denaturation is delayed due to cross-linking. It has also been shown that mechanical stress during heating can delay denaturation and the effect of cross-linking can be compared to t ...
... The control in Fig. 7a refers to protein without any cross-linking. It is observed from the FTIR studies that protein denaturation is delayed due to cross-linking. It has also been shown that mechanical stress during heating can delay denaturation and the effect of cross-linking can be compared to t ...
A1988Q982800002
... obtain distance constraints by experimentalphysical chemical methods to determine the three-dimensional structure of ribonuclease in aqueous solution. For example, three specific Tyr...Asp interactions were identified, and subsequently verified when the crystal structure was determined (see Fig. 5 o ...
... obtain distance constraints by experimentalphysical chemical methods to determine the three-dimensional structure of ribonuclease in aqueous solution. For example, three specific Tyr...Asp interactions were identified, and subsequently verified when the crystal structure was determined (see Fig. 5 o ...
The Structure and Function of Proteins Chapter 5 (continued)
... Lectures by Erin Barley Kathleen Fitzpatrick © 2011 Pearson Education, Inc. ...
... Lectures by Erin Barley Kathleen Fitzpatrick © 2011 Pearson Education, Inc. ...
No Slide Title
... Signal Peptides (2) The common structure of signal peptides from various proteins is described as: • a positively charged (N-terminal) n-region • followed by a hydrophobic h-region (which can adopt an -helical conformation in an hydrophobic environment) • and a neutral but polar c-region (cleavage ...
... Signal Peptides (2) The common structure of signal peptides from various proteins is described as: • a positively charged (N-terminal) n-region • followed by a hydrophobic h-region (which can adopt an -helical conformation in an hydrophobic environment) • and a neutral but polar c-region (cleavage ...
Protein purification
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.The methods used in protein purification can roughly be divided into analytical and preparative methods. The distinction is not exact, but the deciding factor is the amount of protein that can practically be purified with that method. Analytical methods aim to detect and identify a protein in a mixture, whereas preparative methods aim to produce large quantities of the protein for other purposes, such as structural biology or industrial use. In general, the preparative methods can be used in analytical applications, but not the other way around.