WHAT THEY DO
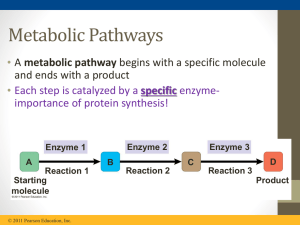
... Enzymes speed up chemical reactions (catalysts) They are required for all biochemical reactions Stay tuned…We will learn more about enzymes later in this week! ...
... Enzymes speed up chemical reactions (catalysts) They are required for all biochemical reactions Stay tuned…We will learn more about enzymes later in this week! ...
2.3 Carbon-Based Molecules TEKS 9A
... The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids ...
... The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids ...
Amino Acids - faculty at Chemeketa
... 2. Why are the triacylglycerols in the intestinal lining coated with proteins to form chylomicrons? The proteins coat the triacylglycerols to make water soluble chylomicrons that move into the lymph and ...
... 2. Why are the triacylglycerols in the intestinal lining coated with proteins to form chylomicrons? The proteins coat the triacylglycerols to make water soluble chylomicrons that move into the lymph and ...
Answers, PS8
... CH908, Problem set 8. Collisionally Activated Dissociation (CAD) of peptides 1. Memorize the structures of the 20 naturally occurring amino acids. ...
... CH908, Problem set 8. Collisionally Activated Dissociation (CAD) of peptides 1. Memorize the structures of the 20 naturally occurring amino acids. ...
chapter 8 notes - 8.4 and 8.5 - APBio09-10
... 6. How is the reaction sped up? a. For 2 or more reactants, the active site provides a template for substrates to come together in the proper orientation b. Enzyme stretches the substrate toward transition state form i. Stretches and bends chemical bonds that must be broken in reaction ii. Ea is dir ...
... 6. How is the reaction sped up? a. For 2 or more reactants, the active site provides a template for substrates to come together in the proper orientation b. Enzyme stretches the substrate toward transition state form i. Stretches and bends chemical bonds that must be broken in reaction ii. Ea is dir ...
Cellular Respiration - Home - Mrs. Guida's AP Biology Class
... Pyruvate Oxidation Krebs Cycle ETC and Chemiosmosis ...
... Pyruvate Oxidation Krebs Cycle ETC and Chemiosmosis ...
4 1. agribiotechnology 2. genetically modified organisms
... 24. The moving units of ATP synthase are 1. a, 2. , 3.c, 4. , 5. (A) 1 and 2. (B) 2, 4, and 5. (C) 3, 4, and 5. (D) 4 and 5. (E) only 2. 25. Which of the following enzymes becomes active when bound to Ca2+ and diacylglycerol. (A) protein kinase A. (B) protein kinase C. (C) phospholipase A1. (D) pho ...
... 24. The moving units of ATP synthase are 1. a, 2. , 3.c, 4. , 5. (A) 1 and 2. (B) 2, 4, and 5. (C) 3, 4, and 5. (D) 4 and 5. (E) only 2. 25. Which of the following enzymes becomes active when bound to Ca2+ and diacylglycerol. (A) protein kinase A. (B) protein kinase C. (C) phospholipase A1. (D) pho ...
Bacterial Genetics
... • Redox potential is a measure of the affinity of compounds for electrons. The more positive a compound’s redox potential is, the greater its tendency to acquire electrons. – Redox potential is measured in millivolts (mV), relative to hydrogen at 1 atm pressure. Compounds are at 1 M concentration. • ...
... • Redox potential is a measure of the affinity of compounds for electrons. The more positive a compound’s redox potential is, the greater its tendency to acquire electrons. – Redox potential is measured in millivolts (mV), relative to hydrogen at 1 atm pressure. Compounds are at 1 M concentration. • ...
PP - Chemistry Courses: About
... • “Glucagon stimulates the liver to generate glucose by glycogenolysis and gluconeogenesis, and it stimulates lipolysis in adipose tissue.” ...
... • “Glucagon stimulates the liver to generate glucose by glycogenolysis and gluconeogenesis, and it stimulates lipolysis in adipose tissue.” ...
Section: Energy and Chemical Reactions
... Energy is the ability to move or change matter. Activation energy is the energy needed to start a chemical reaction. DNA is a nucleic acid that stores hereditary information used to make proteins. RNA is a nucleic acid that is involved in protein synthesis. ATP is an organic molecule that acts as th ...
... Energy is the ability to move or change matter. Activation energy is the energy needed to start a chemical reaction. DNA is a nucleic acid that stores hereditary information used to make proteins. RNA is a nucleic acid that is involved in protein synthesis. ATP is an organic molecule that acts as th ...
Bio 263/F94/T3 V2 - Millersville University
... 44. A molecule is known to bind to calcium ions released into the cytoplasm of a cell causing it to be activated. It then is able to trigger a number of intracellular activities. Of what protein family is this protein likely to be a member? ...
... 44. A molecule is known to bind to calcium ions released into the cytoplasm of a cell causing it to be activated. It then is able to trigger a number of intracellular activities. Of what protein family is this protein likely to be a member? ...
Document
... • If a molecule can bind to another site on the enzyme (besides active site) and stop enzyme function, it is an allosteric inhibitor • Can disrupt the 3D shape of enzyme molecule so active site cannot accept substrate • Can be reversible or irreversible ...
... • If a molecule can bind to another site on the enzyme (besides active site) and stop enzyme function, it is an allosteric inhibitor • Can disrupt the 3D shape of enzyme molecule so active site cannot accept substrate • Can be reversible or irreversible ...
Document
... Now ask yourself "As the pH is raised, which is the first proton to be removed? Is it the proton attached to the positively charged nitrogen, or is it the proton of the carboxyl group?" ...
... Now ask yourself "As the pH is raised, which is the first proton to be removed? Is it the proton attached to the positively charged nitrogen, or is it the proton of the carboxyl group?" ...
Bioinformatic analysis of diverse protein superfamilies to
... Remote evolutionary relatives were superimposed by structural comparison, while sequencebased alignments were assumed meaningful for closer homologs [8]. Systematic bioinformatic analysis of genomic and structural information corresponding to each selected superfamily of enzymes has been carried out ...
... Remote evolutionary relatives were superimposed by structural comparison, while sequencebased alignments were assumed meaningful for closer homologs [8]. Systematic bioinformatic analysis of genomic and structural information corresponding to each selected superfamily of enzymes has been carried out ...
PATHWAYS THAT HARVEST CHEMICAL ENERGY CHAPTER 9
... • Polysaccharides → hydrolyzed to glucose, enters glycolysis and cellular respiration • Lipids → broken down to: glycerol → DAP fatty acids → acetyl CoA • Proteins → hydrolyzed to amino acids—feed into glycolysis or the citric acid cycle ANABOLIC CONVERSIONS • Most catabolic reactions are reversible ...
... • Polysaccharides → hydrolyzed to glucose, enters glycolysis and cellular respiration • Lipids → broken down to: glycerol → DAP fatty acids → acetyl CoA • Proteins → hydrolyzed to amino acids—feed into glycolysis or the citric acid cycle ANABOLIC CONVERSIONS • Most catabolic reactions are reversible ...
COS 3.0 Acids and Bases
... react with acids to form salts. (measures more than 7 on the pH scale) • NEUTRAL - These are items that are neither acids or bases. There are 4 main ways to determine if a substance is and acid or a base. They are: Red litmus paper, Blue litmus paper, pH, and Red Cabbage Juice. ...
... react with acids to form salts. (measures more than 7 on the pH scale) • NEUTRAL - These are items that are neither acids or bases. There are 4 main ways to determine if a substance is and acid or a base. They are: Red litmus paper, Blue litmus paper, pH, and Red Cabbage Juice. ...
Problems in Protein Biosynthesis - The Journal of General Physiology
... messenger RNA (2) complementary to one of the two DNA strands (3, 4). This is catalyzed by RNA polymerase (5-7). The second step will be the topic of this discussion and we will return to it subsequently. The third step is the folding of the peptide chain into a 3-dimensional biologically active for ...
... messenger RNA (2) complementary to one of the two DNA strands (3, 4). This is catalyzed by RNA polymerase (5-7). The second step will be the topic of this discussion and we will return to it subsequently. The third step is the folding of the peptide chain into a 3-dimensional biologically active for ...
Authors` pre-proof version - University of Connecticut
... each class, the aaRSs with different amino acid specificity show distant shared ancestry as revealed by structural, sequence, and enzymatic similarity. However, while nearly all families of aaRS are universally distributed within the three domains (with the exception of some aaRS requiring tRNA-depe ...
... each class, the aaRSs with different amino acid specificity show distant shared ancestry as revealed by structural, sequence, and enzymatic similarity. However, while nearly all families of aaRS are universally distributed within the three domains (with the exception of some aaRS requiring tRNA-depe ...
Proteins - Lectures For UG-5
... the proteins that you eat and digest. Every time you eat a burger (vege or beef), you break the proteins down into single amino acids ready for use in building new proteins. And yes, proteins have the job of digesting proteins, they are known as proteases. There are only 20 different amino acids but ...
... the proteins that you eat and digest. Every time you eat a burger (vege or beef), you break the proteins down into single amino acids ready for use in building new proteins. And yes, proteins have the job of digesting proteins, they are known as proteases. There are only 20 different amino acids but ...
Chorionic gonadotropin (C8554) - Product - Sigma
... β subunit has 145 amino acids. The α subunit is common among the family of glycoprotein hormones; whereas, the hormone-specific β subunit, which exhibits different degrees of homology, may confer biologic specificity of the individual hormone.1 The amino acid sequences of the α subunit5,8 and the β ...
... β subunit has 145 amino acids. The α subunit is common among the family of glycoprotein hormones; whereas, the hormone-specific β subunit, which exhibits different degrees of homology, may confer biologic specificity of the individual hormone.1 The amino acid sequences of the α subunit5,8 and the β ...
ch 7 organic power point
... The water molecule “adds” to the doublebonded carbon atoms by placing an H- on one carbon and an –OH group on the other. H ...
... The water molecule “adds” to the doublebonded carbon atoms by placing an H- on one carbon and an –OH group on the other. H ...
Protein For Athletes
... even the hardest-training athletes, a 154-pound endurance athlete would need no more than 120 grams of protein per day. What are the Differences between Essential and Nonessential Amino Acids? The body can make the majority of the amino acids it needs but lacks the capacity to create all of them, so ...
... even the hardest-training athletes, a 154-pound endurance athlete would need no more than 120 grams of protein per day. What are the Differences between Essential and Nonessential Amino Acids? The body can make the majority of the amino acids it needs but lacks the capacity to create all of them, so ...
1 - Oregon State University
... 1. To be able to list the major types of digestive tract physiology of domestic and wild animals, with particular emphasis on Hofmann’s classification of wild ruminants. Students will be required to describe Hofmann’s categories and explain how anatomical characteristics influence diet selection and ...
... 1. To be able to list the major types of digestive tract physiology of domestic and wild animals, with particular emphasis on Hofmann’s classification of wild ruminants. Students will be required to describe Hofmann’s categories and explain how anatomical characteristics influence diet selection and ...
499 Med Chem Chap 3 problems
... 07) Which of the following descriptions best describes a coenzyme? a. A non-protein substance that is required by an enzyme if it is to catalyse a reaction. b. A non-protein organic molecule that is required by some enzymes in order to catalyse a reaction on a substrate. c. A non-protein organic mol ...
... 07) Which of the following descriptions best describes a coenzyme? a. A non-protein substance that is required by an enzyme if it is to catalyse a reaction. b. A non-protein organic molecule that is required by some enzymes in order to catalyse a reaction on a substrate. c. A non-protein organic mol ...