
Alcohols
... The anion intermediate then expels the –OH group with simultaneous protonation by an acid (HA) to form water ...
... The anion intermediate then expels the –OH group with simultaneous protonation by an acid (HA) to form water ...
Organic Chemistry Fifth Edition
... since abstraction of any of the 8 H atoms results in the same product being formed. ...
... since abstraction of any of the 8 H atoms results in the same product being formed. ...
Homogeneously catalysed hydrogenation of unsaturated fatty acids
... y = x + - a+bx This equation, which represents a hyperbola, allows a simple representation of a complicated chemical reaction and is of practical importance for a systematic study of catalytic processes. It was found that the course of the hydrogenation of unsaturated acids under influence of Cu- an ...
... y = x + - a+bx This equation, which represents a hyperbola, allows a simple representation of a complicated chemical reaction and is of practical importance for a systematic study of catalytic processes. It was found that the course of the hydrogenation of unsaturated acids under influence of Cu- an ...
Origin of the Diastereoselection in the Indium
... that the Z-configuration of the haloallylic sulfones 1 equilibrated to the more stable E-configuration while indium was being inserted. This was presumably the reason that the geometry of the CdC bond of 1 was not important in controlling the stereoselectivity of the In-mediated addition reaction. T ...
... that the Z-configuration of the haloallylic sulfones 1 equilibrated to the more stable E-configuration while indium was being inserted. This was presumably the reason that the geometry of the CdC bond of 1 was not important in controlling the stereoselectivity of the In-mediated addition reaction. T ...
Nucleophilic Substitution and b
... discussed earlier as reverse of hydration Secondary and tertiary alcohols, carbocations Protonation, establishing of good leaving group. Elimination of water to yield carbocation in rate determining step. ...
... discussed earlier as reverse of hydration Secondary and tertiary alcohols, carbocations Protonation, establishing of good leaving group. Elimination of water to yield carbocation in rate determining step. ...
Silicon hydrides in organic synthesis
... solution to 60-8OoC or by irradiating it at 254 nm. To our surprise, we discovered that trace quantities of almost any organic compound containing an oxygen functionality, such as ketones, ethers, esters, etc., have a tremendous catalytic effect on this reaction. In fact, the reaction becomes so rap ...
... solution to 60-8OoC or by irradiating it at 254 nm. To our surprise, we discovered that trace quantities of almost any organic compound containing an oxygen functionality, such as ketones, ethers, esters, etc., have a tremendous catalytic effect on this reaction. In fact, the reaction becomes so rap ...
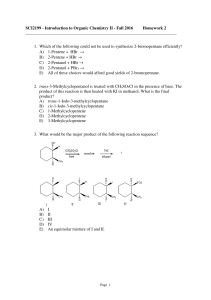
SCI2199 - Introduction to Organic Chemistry II
... product of the reaction then is allowed to react with KI in methanol. What is the final product? A) trans-1-Iodo-3-methylcyclopentane B) cis-1-Iodo-3-methylcyclopentane C) 1-Methylcyclopentene D) 2-Methylcyclopentene E) 3-Methylcyclopentene 10. The reaction between 4-methyl-1-pentanol and PBr3 to yi ...
... product of the reaction then is allowed to react with KI in methanol. What is the final product? A) trans-1-Iodo-3-methylcyclopentane B) cis-1-Iodo-3-methylcyclopentane C) 1-Methylcyclopentene D) 2-Methylcyclopentene E) 3-Methylcyclopentene 10. The reaction between 4-methyl-1-pentanol and PBr3 to yi ...
Şenol, O.İ., Viljava, T.-R., Krause, AOI
... Dehydration of alcohols yields the alkenes and hydrogenation of alkenes leads to the corresponding alkane. In path II, the deesterification reaction yields carboxylic acids and methanol. The water required for the reaction may be supplied by the dehydration of the alcohols in path I. The formed carb ...
... Dehydration of alcohols yields the alkenes and hydrogenation of alkenes leads to the corresponding alkane. In path II, the deesterification reaction yields carboxylic acids and methanol. The water required for the reaction may be supplied by the dehydration of the alcohols in path I. The formed carb ...
Reactions of Alcohol
... Reactions of Alcohols In biological reactions the coenzyme NAD+ is often used as the oxidizing agent. The NAD+ takes the ...
... Reactions of Alcohols In biological reactions the coenzyme NAD+ is often used as the oxidizing agent. The NAD+ takes the ...
Alkynes
... But typical of synthetic problems side reaction occurs to some extent and must be taken into account. ...
... But typical of synthetic problems side reaction occurs to some extent and must be taken into account. ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition
... • PLP is an aldehyde that readily forms imines from amino groups of substrates, such as amino acids • The imine undergoes a proton shift that leads to the net conversion of the amino group of the substrate into a carbonyl group ...
... • PLP is an aldehyde that readily forms imines from amino groups of substrates, such as amino acids • The imine undergoes a proton shift that leads to the net conversion of the amino group of the substrate into a carbonyl group ...
Mass Spec - Fragmentation
... It is usually easy to see the M+ peak of alkenes in EIMS. In acyclic alkenes, the double bond freely migrates in the fragments, so it can be difficult to determine the double bond location, but for cyclic alkenes it is easier. Cleavage usually happens at allylic bonds (Rule 5). ...
... It is usually easy to see the M+ peak of alkenes in EIMS. In acyclic alkenes, the double bond freely migrates in the fragments, so it can be difficult to determine the double bond location, but for cyclic alkenes it is easier. Cleavage usually happens at allylic bonds (Rule 5). ...
Chapter 1 Structure and Bonding
... Protonation from solvent yields the product The new Nu—C bond has both electrons from Nu- (like in SN2) An electron pair is the “leaving group” Strongly basic nucleophiles typically follow this mechanism ...
... Protonation from solvent yields the product The new Nu—C bond has both electrons from Nu- (like in SN2) An electron pair is the “leaving group” Strongly basic nucleophiles typically follow this mechanism ...
Lectures 15, 16 and 17
... 19.22) Comparing CF3SO3H and CH3SO3H, which has the weaker conjugate base? Which conjugate base is the better leaving group? Which of these acids has the higher pka? CF3SO3H is the weaker conjugate base. CF3SO3H is the better leaving group because it is the weaker conjugate base. CH3SO3H, with the ...
... 19.22) Comparing CF3SO3H and CH3SO3H, which has the weaker conjugate base? Which conjugate base is the better leaving group? Which of these acids has the higher pka? CF3SO3H is the weaker conjugate base. CF3SO3H is the better leaving group because it is the weaker conjugate base. CH3SO3H, with the ...
Full Article-PDF - UNC
... The selective manipulation of functional groups in complex organic molecules often requires the use of protecting groups. While a variety of useful hydroxyl protecting groups are available, l, 2 occasionally, a situation arises which cannot be resolved by existing protecting group technology. During ...
... The selective manipulation of functional groups in complex organic molecules often requires the use of protecting groups. While a variety of useful hydroxyl protecting groups are available, l, 2 occasionally, a situation arises which cannot be resolved by existing protecting group technology. During ...
Alcohols - Chem1-tsu
... In each addition step, the boron atom is attached to the sp2 carbon atom that is bonded to greater number of hydrogen atoms. The hydrogen atom of the boron atom attaches to the other carbon of the double bond. Thus this is anti-Markovnikov's addition. During the oxidation of trialkyl borane, boron ...
... In each addition step, the boron atom is attached to the sp2 carbon atom that is bonded to greater number of hydrogen atoms. The hydrogen atom of the boron atom attaches to the other carbon of the double bond. Thus this is anti-Markovnikov's addition. During the oxidation of trialkyl borane, boron ...
Review
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
aciee-2004-43-5442-palomo
... of the fluoride-catalyzed reaction of silyl nitronates and aldehydes. Only quite recently—almost 25 years later—two independent groups have developed chiral catalysts.[10] Maruoka et al.[10a] have reported the addition of trimethylsilyl nitronates 2 to aromatic aldehydes 1 in the presence of 2 mol % ...
... of the fluoride-catalyzed reaction of silyl nitronates and aldehydes. Only quite recently—almost 25 years later—two independent groups have developed chiral catalysts.[10] Maruoka et al.[10a] have reported the addition of trimethylsilyl nitronates 2 to aromatic aldehydes 1 in the presence of 2 mol % ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to
... equilibrium mixture containing the corresponding hemiacetal è A hemiacetal has a hydroxyl and alkoxyl group on the same carbon è Acylic hemiacetals are generally not stable, however, cyclic five- and sixmembered ring hemiacetals are ...
... equilibrium mixture containing the corresponding hemiacetal è A hemiacetal has a hydroxyl and alkoxyl group on the same carbon è Acylic hemiacetals are generally not stable, however, cyclic five- and sixmembered ring hemiacetals are ...
Notes 07 Organometallic Compounds with notes
... 1. What is the oxidation state change for the carbon in the reaction methyl iodide to methyl lithium? Reduction: -2 to -4. 2. What is the oxidation state change for the lithium in the reaction methyl iodide to methyl lithium? Oxidation: +1 to 0. 3. What is the oxidation state change for the iodide i ...
... 1. What is the oxidation state change for the carbon in the reaction methyl iodide to methyl lithium? Reduction: -2 to -4. 2. What is the oxidation state change for the lithium in the reaction methyl iodide to methyl lithium? Oxidation: +1 to 0. 3. What is the oxidation state change for the iodide i ...
Reduction [H]
... Thioacetal (or thioketal) reduction or Mozingo reduction with Raney nickel and hydrogen is a classic method to prepare a methylene group from a carbonyl compound. Raney Nickel was developed in 1926 by American engineer Murray Raney. ( "Method of producing finely-divided nickel,"U.S. patent 1,628,190 ...
... Thioacetal (or thioketal) reduction or Mozingo reduction with Raney nickel and hydrogen is a classic method to prepare a methylene group from a carbonyl compound. Raney Nickel was developed in 1926 by American engineer Murray Raney. ( "Method of producing finely-divided nickel,"U.S. patent 1,628,190 ...
10_09_11.html
... Kinetic control: major product is the one formed at the fastest rate Thermodynamic control: major product is the one that is the most stable ...
... Kinetic control: major product is the one formed at the fastest rate Thermodynamic control: major product is the one that is the most stable ...
Lecture 1: Key Concepts in Stereoselective Synthesis
... Reviews have stated that the hydroamination of olefins is thermodynamically favored. However, experimental equilibrium constants, enthalpies, and entropies for olefin hydroamination in solution are lacking. In 2006, Hartwig reported direct measurements of the equilibrium constants for addition of ar ...
... Reviews have stated that the hydroamination of olefins is thermodynamically favored. However, experimental equilibrium constants, enthalpies, and entropies for olefin hydroamination in solution are lacking. In 2006, Hartwig reported direct measurements of the equilibrium constants for addition of ar ...
Kinetic resolution

In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. This enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes. Kinetic resolution is a concept in organic chemistry and can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts are much less common than the use of enzymatic kinetic resolution in application towards organic synthesis, although a number of useful synthetic techniques have been developed in the past 30 years.



















![Reduction [H]](http://s1.studyres.com/store/data/007148356_1-25f5210a5c809c157bb6e0d32d66fd9d-300x300.png)


