Inorganic Chemistry of LanthanideIV:
... catalytic converters in automotive applications. Cerium dioxide is able to release or store oxygen in the exhaust stream of an automotive engine because the material is able to become non-stoichiometric in its oxygen-content. The catalytic activity of ceria has been found to depend directly on the n ...
... catalytic converters in automotive applications. Cerium dioxide is able to release or store oxygen in the exhaust stream of an automotive engine because the material is able to become non-stoichiometric in its oxygen-content. The catalytic activity of ceria has been found to depend directly on the n ...
$doc.title
... suffix -‐yl from the root of the carboxylic acid – CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl ...
... suffix -‐yl from the root of the carboxylic acid – CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl ...
Document
... EDTA Titrations Metal Chelate Complexes – complexes formed between a Lewis acid and a Lewis base. Metals ions are Lewis acids, because they accept electrons from Lewis bases. When metal cations combine with Lewis bases, the resulting species is called a complex ion, and the base is called a ligand. ...
... EDTA Titrations Metal Chelate Complexes – complexes formed between a Lewis acid and a Lewis base. Metals ions are Lewis acids, because they accept electrons from Lewis bases. When metal cations combine with Lewis bases, the resulting species is called a complex ion, and the base is called a ligand. ...
New. J. Chem., 1992, 16, 633-642
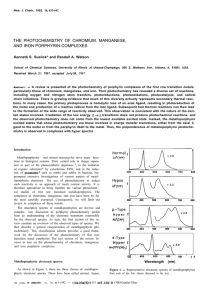
... The photoreductions of both porphyrins and metalloporphyrins are also known. In these cases reducing agents such as ascorbic acid, glutathione, EDTA, or ethyl acetoacetonate are necessary “. In strongly acidic solutions, porphyrins can be rapidly photoreduced to chlorins and bacteriochlorins. These ...
... The photoreductions of both porphyrins and metalloporphyrins are also known. In these cases reducing agents such as ascorbic acid, glutathione, EDTA, or ethyl acetoacetonate are necessary “. In strongly acidic solutions, porphyrins can be rapidly photoreduced to chlorins and bacteriochlorins. These ...
Phosphorescent Organic Light-Emitting Devices: Working Principle and Iridium Based Emitter Materials
... luminescent compounds exhibits only weak spin-orbit couplings rendering these small molecules and polymers fluorescent with negligible radiative rates from triplet states. Competing nonradiative processes (e.g. triplet-triplet annihilation or vibronic relaxation) effectively quench the phosphorescen ...
... luminescent compounds exhibits only weak spin-orbit couplings rendering these small molecules and polymers fluorescent with negligible radiative rates from triplet states. Competing nonradiative processes (e.g. triplet-triplet annihilation or vibronic relaxation) effectively quench the phosphorescen ...
Chapter 2 - University of Amsterdam
... Using high-pressure IR spectroscopy we studied the formation of complexes cis[Rh(H)(CO)31a] and trans-[Rh(H)(CO)31a(2)2] under actual catalytic conditions, using 20 bar of syngas (H2/CO 1:1) and concentrations identical to those in catalysis experiments.12 In the presence of [Rh(acac)CO2] and ligand ...
... Using high-pressure IR spectroscopy we studied the formation of complexes cis[Rh(H)(CO)31a] and trans-[Rh(H)(CO)31a(2)2] under actual catalytic conditions, using 20 bar of syngas (H2/CO 1:1) and concentrations identical to those in catalysis experiments.12 In the presence of [Rh(acac)CO2] and ligand ...
Organometallic synthesis, reactivity and catalysis in the solid state
... Rh(III) complex [Rh( Bu2 PCH2 CH2 P Bu2 )(Cl){(C6 H3 Cl)BAr3 }]2 [59]. Interestingly, this process generates two isomers in the solid state, whereas the same process in solution only accesses the thermodynamic isomer. Bond isomerizations in the solid state, involving C−C cleavage, between Ru-alkynyl ...
... Rh(III) complex [Rh( Bu2 PCH2 CH2 P Bu2 )(Cl){(C6 H3 Cl)BAr3 }]2 [59]. Interestingly, this process generates two isomers in the solid state, whereas the same process in solution only accesses the thermodynamic isomer. Bond isomerizations in the solid state, involving C−C cleavage, between Ru-alkynyl ...
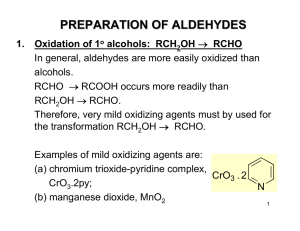
PREPARATION OF ALDEHYDES
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
Title Iridium, ruthenium, and palladium complexes containing a
... imidazo[1,2-a]pyridinium salt 1 as a pale yellow solid. Iridium and ruthenium complexation. Metallation of 1 via C–H bond activation using [IrCp*Cl2]2 in refluxing toluene did not produce any carbene complex and just returned starting materials. As a consequence, the silver carbene formation and sub ...
... imidazo[1,2-a]pyridinium salt 1 as a pale yellow solid. Iridium and ruthenium complexation. Metallation of 1 via C–H bond activation using [IrCp*Cl2]2 in refluxing toluene did not produce any carbene complex and just returned starting materials. As a consequence, the silver carbene formation and sub ...
Synthesis, characterization and spectroscopic investigation
... indicate the absence of water molecules in these systems, indicating that these coordination compounds are anhydrous, corroborating with the infrared data. Based on the X-ray diffraction patterns (Fig. 2), the three thulium complexes present high crystallinity. However, the data show that they are n ...
... indicate the absence of water molecules in these systems, indicating that these coordination compounds are anhydrous, corroborating with the infrared data. Based on the X-ray diffraction patterns (Fig. 2), the three thulium complexes present high crystallinity. However, the data show that they are n ...
1.Isomerism Notes
... pairs. For example, in NO2 ion, the nitrogen atom as well as the oxygen atom can donate lone pairs. This gives rise to isomerism. If nitrogen donates its lone pair, one particular compound will be formed. If oxygen does so, a different compound (although having the same molecular formula) is obtaine ...
... pairs. For example, in NO2 ion, the nitrogen atom as well as the oxygen atom can donate lone pairs. This gives rise to isomerism. If nitrogen donates its lone pair, one particular compound will be formed. If oxygen does so, a different compound (although having the same molecular formula) is obtaine ...
Aldehydes and Ketones - Belle Vernon Area School District
... Reduction of aldes and kets to alcohols O R-C-H + H2 –Pt/Pd R-C-OH RCHO + H2 –Pt/Pd RCH2OH O O R-C-R + H2 –Pt/Pd R-C-R RCOR + H2 –Pt/Pd RCHOHR ...
... Reduction of aldes and kets to alcohols O R-C-H + H2 –Pt/Pd R-C-OH RCHO + H2 –Pt/Pd RCH2OH O O R-C-R + H2 –Pt/Pd R-C-R RCOR + H2 –Pt/Pd RCHOHR ...
lecture 6 oxidative addition
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
Stabilization and Reactivity of Low Oxidation State Indium Compounds
... 2.1 Amounts of InX taken into ether or toluene solution as determined by ICPMS. All values are given in %. All values reported represent the average of the triplicate trials. Missing values indicate disproportionation occurred. The complete set of data can be found in the supporting information..... ...
... 2.1 Amounts of InX taken into ether or toluene solution as determined by ICPMS. All values are given in %. All values reported represent the average of the triplicate trials. Missing values indicate disproportionation occurred. The complete set of data can be found in the supporting information..... ...
The coordination chemistry of pyridyl oximes
... The coordination chemistry of pyridyl oximes is reviewed. Simple pyridyl oximes have the general formula (py)C(R)NOH, where py is a pyridyl group (2-, 3- or 4-) attached to the oxime carbon atom and R can be a donor or a non-donor group. There are also ligands containing more pyridyl and/or oxime gr ...
... The coordination chemistry of pyridyl oximes is reviewed. Simple pyridyl oximes have the general formula (py)C(R)NOH, where py is a pyridyl group (2-, 3- or 4-) attached to the oxime carbon atom and R can be a donor or a non-donor group. There are also ligands containing more pyridyl and/or oxime gr ...
Ruthenium(II/III) - Publications of the IAS Fellows
... higher energy bands in the UV region are of intra-ligand p–p* type or charge-transfer transitions involving energy levels which are higher in energy than the ligand lowest unoccupied molecular orbital (LUMO). It may be noted that for the Ru(bpy) 21 complex the ...
... higher energy bands in the UV region are of intra-ligand p–p* type or charge-transfer transitions involving energy levels which are higher in energy than the ligand lowest unoccupied molecular orbital (LUMO). It may be noted that for the Ru(bpy) 21 complex the ...
LECTURE 7 REDUCTIVE ELIMINATIONSa
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
Epoxidation of α-pinene mediated by cobalt(III) catalysts
... conversion of 81.4% with a turnover frequency (TOF) of 105. ...
... conversion of 81.4% with a turnover frequency (TOF) of 105. ...
Basico- Teoria_Campo-Cristalino-Shriver-Atkins
... field. Figure 20.5 shows the case of a d6 ion. In both the free ion and the high-spin complex two electrons are paired, whereas in the low-spin case all six electrons occur as three pairs. Thus we do not need to consider the pairing energy in the high-spin case, as there is no additional pairing. Th ...
... field. Figure 20.5 shows the case of a d6 ion. In both the free ion and the high-spin complex two electrons are paired, whereas in the low-spin case all six electrons occur as three pairs. Thus we do not need to consider the pairing energy in the high-spin case, as there is no additional pairing. Th ...
(PCP)Ir(O2)_BW_9-25.dot - Werner Kaminsky
... Iridium pincer hydride complexes (tBuPCP)Ir(H)(X) [X = Ph (2), H (3), OH (4) and CCPh (5)] react with O2 in benzene solution to give the peroxo complexes ( tBuPCP)Ir(O2)n [tBuPCP = 3-C6H3(CH2PtBu2)2; n = 1 (6), 2 (7)]. The bis(peroxo) complex 7 has been characterized by an X-ray crystal structure. ...
... Iridium pincer hydride complexes (tBuPCP)Ir(H)(X) [X = Ph (2), H (3), OH (4) and CCPh (5)] react with O2 in benzene solution to give the peroxo complexes ( tBuPCP)Ir(O2)n [tBuPCP = 3-C6H3(CH2PtBu2)2; n = 1 (6), 2 (7)]. The bis(peroxo) complex 7 has been characterized by an X-ray crystal structure. ...
8 reactions of a cetylenes
... accept electron density from filled metal π-orbitals. The simplest situation, π-bonding to a single metal atom is shown in structure tin Figure 8-1. The presence of two mutually orthogonal π-systems in the acetylene bond also permits simultaneous πbonding to two metal atoms. This situation, which i ...
... accept electron density from filled metal π-orbitals. The simplest situation, π-bonding to a single metal atom is shown in structure tin Figure 8-1. The presence of two mutually orthogonal π-systems in the acetylene bond also permits simultaneous πbonding to two metal atoms. This situation, which i ...
Richard R. Schrock - Nobel Lecture
... was intrigued by high oxidation state peralkyl complexes and had chosen to explore the organometallic chemistry of tantalum soon after my arrival at DuPont. Little alkyl chemistry was known of the metals in group 5 (V, Nb, Ta) at that time; I chose tantalum because it is next to tungsten in group 6 ...
... was intrigued by high oxidation state peralkyl complexes and had chosen to explore the organometallic chemistry of tantalum soon after my arrival at DuPont. Little alkyl chemistry was known of the metals in group 5 (V, Nb, Ta) at that time; I chose tantalum because it is next to tungsten in group 6 ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.