PDF File
... on the nucleophile (Fig. 5.2B), and on the nonbridging phosphoryl oxygen atom (Fig. 5.2C). Further, they may coordinate both the nucleophile and the nonbridging phosphoryl oxygen (Fig. 5.2D), stabilizing the in-line geometry required for phosphoryl transfer reactions. In addition, metal ions may imp ...
... on the nucleophile (Fig. 5.2B), and on the nonbridging phosphoryl oxygen atom (Fig. 5.2C). Further, they may coordinate both the nucleophile and the nonbridging phosphoryl oxygen (Fig. 5.2D), stabilizing the in-line geometry required for phosphoryl transfer reactions. In addition, metal ions may imp ...
The effect of halide ions on nickel corrosion in perchloric acid solutions
... with nickel-containing items, while in others it breaks out only after many years of skin contact with nickel. Sometimes it is due to contact with metal items containing nickel, but often there is no obvious reason for it. It has been suggested that in some persons dyshidrotic hand dermatitis is due ...
... with nickel-containing items, while in others it breaks out only after many years of skin contact with nickel. Sometimes it is due to contact with metal items containing nickel, but often there is no obvious reason for it. It has been suggested that in some persons dyshidrotic hand dermatitis is due ...
Mono- and Trinuclear Nickel(II) Complexes with Sulfur-Containing Oxime Ligands:
... donor atoms to form oligonuclear compounds.6,7 The attendant magnetic exchange interactions have recently also drawn some attention.8,9 Results and Discussion Synthesis. The ligand was prepared according to Scheme 1 using two alternative routes. In route (i), the order of combining the reagents is q ...
... donor atoms to form oligonuclear compounds.6,7 The attendant magnetic exchange interactions have recently also drawn some attention.8,9 Results and Discussion Synthesis. The ligand was prepared according to Scheme 1 using two alternative routes. In route (i), the order of combining the reagents is q ...
Influence of Substituents on the Energy and Nature of the Lowest
... over the whole visible range, it is highly desirable to find a way to modify the HOMO and LUMO energies independently. When the molecular complex possesses different chromophoric ligands, some caution is required in determining the “optical” HOMO-LUMO gap, i.e., the gap between the orbitals involved ...
... over the whole visible range, it is highly desirable to find a way to modify the HOMO and LUMO energies independently. When the molecular complex possesses different chromophoric ligands, some caution is required in determining the “optical” HOMO-LUMO gap, i.e., the gap between the orbitals involved ...
Substituent Effect in the γ–Position of Acetylacetonate on the
... equatorial ligand field strength. In other words, presence ...
... equatorial ligand field strength. In other words, presence ...
Monomer
... → The carbons at the end of the polymer chain can have optical activity : Population is very negligible → Other carbons : not much optical activity ∴ The sum of optical activity is below the limits of detection ...
... → The carbons at the end of the polymer chain can have optical activity : Population is very negligible → Other carbons : not much optical activity ∴ The sum of optical activity is below the limits of detection ...
hydrides and mononuclear rhodium(II) complexes
... three limiting forms, i.e. {RhI + H+ }, {RhII + H• }, and {RhIII + H− }. A number of intermediates and oxidation states have been generated and explored in kinetic and mechanistic studies. Monomeric macrocyclic rhodium(II) complexes, such as L(H2 O)Rh2+ (L = L1 = [14]aneN4 , or L2 = mesoMe6 [14]aneN ...
... three limiting forms, i.e. {RhI + H+ }, {RhII + H• }, and {RhIII + H− }. A number of intermediates and oxidation states have been generated and explored in kinetic and mechanistic studies. Monomeric macrocyclic rhodium(II) complexes, such as L(H2 O)Rh2+ (L = L1 = [14]aneN4 , or L2 = mesoMe6 [14]aneN ...
Some recent coordination chemistry of lead(ll)
... expansive literature dealing with macrocyclic ligands is treated in a separate section as it merits being considered as a "special case" of coordination chemistry. O f all p-block elements, lead(II) has a particular fascination for coordination chemists, as it can adopt many different geometries in ...
... expansive literature dealing with macrocyclic ligands is treated in a separate section as it merits being considered as a "special case" of coordination chemistry. O f all p-block elements, lead(II) has a particular fascination for coordination chemists, as it can adopt many different geometries in ...
as a PDF
... The results of the two speciation methods applied to test solutions and theoretically computed inorganic speciations are shown in Fig. 3. The speciation of the 10 -2 M CaCI 2 solution gave the expected results. The ED method determined 100% low molecular weight Cd. This is in accordance with the fac ...
... The results of the two speciation methods applied to test solutions and theoretically computed inorganic speciations are shown in Fig. 3. The speciation of the 10 -2 M CaCI 2 solution gave the expected results. The ED method determined 100% low molecular weight Cd. This is in accordance with the fac ...
Synthesis of chiral furanoside diphosphinite and thioether-phosphinite compounds derived from
... steps. The solution structures of the trigonal bipyramidal hydrodorhodium complexes with bidentate phosphorus ligand (P-P) [HRh(P-P)(CO)2], which are the resting states in the hydroformylation reaction, have been analyzed in detail.xi These complexes are generally assumed to have a trigonal bipyrami ...
... steps. The solution structures of the trigonal bipyramidal hydrodorhodium complexes with bidentate phosphorus ligand (P-P) [HRh(P-P)(CO)2], which are the resting states in the hydroformylation reaction, have been analyzed in detail.xi These complexes are generally assumed to have a trigonal bipyrami ...
Main Group Metallocenes - Macdonald Research Group
... Cp*Ga and Cp*In form isostructural hexameric clusters with S6 symmetry in the solid state the structures are determined by the close packing of the ligands. Note that these clusters do not conform to Wade’s rules that you would use for boranes. ...
... Cp*Ga and Cp*In form isostructural hexameric clusters with S6 symmetry in the solid state the structures are determined by the close packing of the ligands. Note that these clusters do not conform to Wade’s rules that you would use for boranes. ...
P-block Cyclopentadienyl Compounds
... Cp*Ga and Cp*In form isostructural hexameric clusters with S6 symmetry in the solid state the structures are determined by the close packing of the ligands. Note that these clusters do not conform to Wade’s rules that you would use for boranes. ...
... Cp*Ga and Cp*In form isostructural hexameric clusters with S6 symmetry in the solid state the structures are determined by the close packing of the ligands. Note that these clusters do not conform to Wade’s rules that you would use for boranes. ...
From nucleotides to ribozymes — A comparison of their metal ion
... Any RNA or nucleic acid is a polyanion due to the negatively charged phosphate-sugar backbone. Nevertheless, large RNAs fold to very complex three-dimensional structures containing not only A-form helices but also loops, bulges, and many other local structures, which form a multitude of tertiary con ...
... Any RNA or nucleic acid is a polyanion due to the negatively charged phosphate-sugar backbone. Nevertheless, large RNAs fold to very complex three-dimensional structures containing not only A-form helices but also loops, bulges, and many other local structures, which form a multitude of tertiary con ...
lecture 7 reductive eliminations
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
... • The reverse reaction, reductive elimination, leads to the extrusion of A−B from an M(A)(B) complex and is often the product‐forming step in a catalytic reaction. • In the oxidative addition direction, we break the A−B bond and form an M−A and an M−B bond. • The oxidation state (OS), electron count ...
Hydrolysis Reactions
... A water molecule has two lone pairs of electrons on the oxygen atom, so therefore water can behave as an electron pair donor (Lewis base or ligand). When a transition metal ion is placed in water, it becomes surrounded by six water molecules, each of which donates one electron pair to the metal ion, ...
... A water molecule has two lone pairs of electrons on the oxygen atom, so therefore water can behave as an electron pair donor (Lewis base or ligand). When a transition metal ion is placed in water, it becomes surrounded by six water molecules, each of which donates one electron pair to the metal ion, ...
Lecture 11 – Reaction Types and Mechanisms for
... The process repeats itself until the hexaaquo ion is formed. Each of these mechanisms involves a reaction to give a reactive intermediate with a different coordination number (5 or 7). When M = Co(III) (low-spin d6), considerable crystal field stabilization is lost because the CFSE is so much greate ...
... The process repeats itself until the hexaaquo ion is formed. Each of these mechanisms involves a reaction to give a reactive intermediate with a different coordination number (5 or 7). When M = Co(III) (low-spin d6), considerable crystal field stabilization is lost because the CFSE is so much greate ...
Roald Hoffmann - Nobel Lecture
... analogous to 37 is certainly not much higher in energy than 38, but nevertheless the lowest energy structure is bridged. Movement of some ligands (e.g. carbonyls, but not phosphines) in and out of bridging sites is an experimental reality, a facile process, for transition metal complexes, especially ...
... analogous to 37 is certainly not much higher in energy than 38, but nevertheless the lowest energy structure is bridged. Movement of some ligands (e.g. carbonyls, but not phosphines) in and out of bridging sites is an experimental reality, a facile process, for transition metal complexes, especially ...
building bridges between inorganic and organic chemistry
... analogous to 37 is certainly not much higher in energy than 38, but nevertheless the lowest energy structure is bridged. Movement of some ligands (e.g. carbonyls, but not phosphines) in and out of bridging sites is an experimental reality, a facile process, for transition metal complexes, especially ...
... analogous to 37 is certainly not much higher in energy than 38, but nevertheless the lowest energy structure is bridged. Movement of some ligands (e.g. carbonyls, but not phosphines) in and out of bridging sites is an experimental reality, a facile process, for transition metal complexes, especially ...
2 - Quantum Chemistry Group
... • Cube surrounding Mn2+ is distortedTd symmetry! • But no distortion when BaF2 is changed by CaF2 or SrF2 • What is the origin if there is not a Jahn-Teller effect? • Why at T>50K the system appears as cubic? Phase transition? ...
... • Cube surrounding Mn2+ is distortedTd symmetry! • But no distortion when BaF2 is changed by CaF2 or SrF2 • What is the origin if there is not a Jahn-Teller effect? • Why at T>50K the system appears as cubic? Phase transition? ...
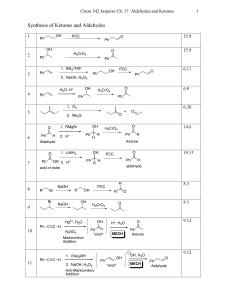
Synthesis of Ketones and Aldehydes
... • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict what product forms that can be isolated, you will need to know when an add ...
... • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict what product forms that can be isolated, you will need to know when an add ...
“Synthetic Mononuclear Nonheme Iron
... CONSPECTUS: Mononuclear nonheme iron−oxygen species, such as iron− superoxo, −peroxo, −hydroperoxo, and −oxo, are key intermediates involved in dioxygen activation and oxidation reactions catalyzed by nonheme iron enzymes. Because these iron−oxygen intermediates are short-lived due to their thermal ...
... CONSPECTUS: Mononuclear nonheme iron−oxygen species, such as iron− superoxo, −peroxo, −hydroperoxo, and −oxo, are key intermediates involved in dioxygen activation and oxidation reactions catalyzed by nonheme iron enzymes. Because these iron−oxygen intermediates are short-lived due to their thermal ...
Topic 15 notes - A
... These are known as multidentate ligands. Examples are ethanedioate (C2O42-) and 1,2diaminoethane (H2NCH2CH2NH2), both of which donate 2 lone pairs per ligand and are said to be bidentate. One unusual ligand, known as edta4-, can form 6 dative covalent bonds per ligand. It is thus said to be hexadent ...
... These are known as multidentate ligands. Examples are ethanedioate (C2O42-) and 1,2diaminoethane (H2NCH2CH2NH2), both of which donate 2 lone pairs per ligand and are said to be bidentate. One unusual ligand, known as edta4-, can form 6 dative covalent bonds per ligand. It is thus said to be hexadent ...
Computational Evidence of the Importance of Substituent Bulk on
... Following their initial discovery1 and subsequent categorization,2 agostic interactions have been found to be extremely common, provided a metal has a low-lying empty valence orbital. In fact, there has been little scrutiny of what factors can assist in stabilizing agostic interactions. Every comple ...
... Following their initial discovery1 and subsequent categorization,2 agostic interactions have been found to be extremely common, provided a metal has a low-lying empty valence orbital. In fact, there has been little scrutiny of what factors can assist in stabilizing agostic interactions. Every comple ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.