and Nickel(II) Chlorides
... bonded acrylamide complexes have been fully characterized by spectroscopic and single crystal X-ray diffraction studies. Savost’yanov et al. and Evastratova et al. have published the syntheses and single X-ray crystal structures of CoII [9] and CuII [11] nitrates with O-bound acrylamide to the metal ...
... bonded acrylamide complexes have been fully characterized by spectroscopic and single crystal X-ray diffraction studies. Savost’yanov et al. and Evastratova et al. have published the syntheses and single X-ray crystal structures of CoII [9] and CuII [11] nitrates with O-bound acrylamide to the metal ...
06_chapter 1
... for partial oxidation of methane. Even though much research has been done in last two decades, no industrial technology has been established. Catalyst deactivation due to coke formation is the main hurdle to overcome, and the use of noble metals was a choice to reduce the deactivation [30]. But, the ...
... for partial oxidation of methane. Even though much research has been done in last two decades, no industrial technology has been established. Catalyst deactivation due to coke formation is the main hurdle to overcome, and the use of noble metals was a choice to reduce the deactivation [30]. But, the ...
Dimethylsulphoxide Complexes of Vanadium(III) o
... bonded to the metal ion. The peak at around 285 cm- I is assigned for the M-Q-S angle deformation as reported for the [Cr(DMSO)]," species (Berney and Weber 1968) and the bands at 275·205 cm- l region to CH 3 torsion modes. Although bands below 100 cm- 1 , assigned to coordinated DMSO have been repo ...
... bonded to the metal ion. The peak at around 285 cm- I is assigned for the M-Q-S angle deformation as reported for the [Cr(DMSO)]," species (Berney and Weber 1968) and the bands at 275·205 cm- l region to CH 3 torsion modes. Although bands below 100 cm- 1 , assigned to coordinated DMSO have been repo ...
Revised_for_DT_final-1 - Pure
... Metal vinylidene complexes are widely encountered, or postulated, as intermediates in a range of important metalmediated transformations of alkynes. However, fluorovinylidene complexes have rarely been described and their reactivity is largely unexplored. By making use of the novel outer-sphere elec ...
... Metal vinylidene complexes are widely encountered, or postulated, as intermediates in a range of important metalmediated transformations of alkynes. However, fluorovinylidene complexes have rarely been described and their reactivity is largely unexplored. By making use of the novel outer-sphere elec ...
aldehydes and ketones
... Step II : In ammonia derivatives, the nitrogen atom has a lone pair of electrons, which attack the positively charged carbonyl carbon and results in positive charge on nitrogen atom ...
... Step II : In ammonia derivatives, the nitrogen atom has a lone pair of electrons, which attack the positively charged carbonyl carbon and results in positive charge on nitrogen atom ...
The Role of Metal Ions in RNA Biochemistry
... particularly important during the process of RNA folding, as the negatively charged backbones from two or more regions of the primary sequence most come close together in space. Without cations to screen these charges, the repulsive forces generated in the close-packed structure would overwhelm the ...
... particularly important during the process of RNA folding, as the negatively charged backbones from two or more regions of the primary sequence most come close together in space. Without cations to screen these charges, the repulsive forces generated in the close-packed structure would overwhelm the ...
C-H and H-H Activation in Transition Metal Complexes and on
... is represented by a band of occupied levels and a band of unoccupied ones. The metal surface will be no different. One of the metal-filled levels (Don for Donor) and one of the unfilled ones (Acc for Acceptor) is singled out, for reasons which will soon become apparent. At the end of the reaction tw ...
... is represented by a band of occupied levels and a band of unoccupied ones. The metal surface will be no different. One of the metal-filled levels (Don for Donor) and one of the unfilled ones (Acc for Acceptor) is singled out, for reasons which will soon become apparent. At the end of the reaction tw ...
Coordination Compounds (NCERT)
... coordination number, coordination polyhedron, homoleptic and heteroleptic. Answer (i) Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number ...
... coordination number, coordination polyhedron, homoleptic and heteroleptic. Answer (i) Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number ...
CHAPTER 1 INTRODUCTION 1.1 HYDRAZINE
... powered engines. As a strong reducing agent, hydrazine is used for corrosion control in boilers and hot water heating systems, for metal plating, and for reducing noble metal catalysts and unsaturated bonds in organic compounds. It is also an oxidizing agent under suitable conditions with two active ...
... powered engines. As a strong reducing agent, hydrazine is used for corrosion control in boilers and hot water heating systems, for metal plating, and for reducing noble metal catalysts and unsaturated bonds in organic compounds. It is also an oxidizing agent under suitable conditions with two active ...
Infrared spectroscopic studies of the different solid
... cobalt(II), zinc(II) and cadmium(II) salts with urea at boiling point of water solvent (100 C) were obtained. The spectra of free urea ligand, manganese(II), cobalt(II), zinc(II) and cadmium(II) carbonates are shown in Fig. 1-5, respectively. The infrared spectra show no bands due to any of the reac ...
... cobalt(II), zinc(II) and cadmium(II) salts with urea at boiling point of water solvent (100 C) were obtained. The spectra of free urea ligand, manganese(II), cobalt(II), zinc(II) and cadmium(II) carbonates are shown in Fig. 1-5, respectively. The infrared spectra show no bands due to any of the reac ...
Chapter 24 Chemistry of Coordination Compounds
... Now, think of point charges being attracted to metal nucleus Positive charge. What about electrons in d orbitals? Ligand negative charge Is repelled by d electrons, ...
... Now, think of point charges being attracted to metal nucleus Positive charge. What about electrons in d orbitals? Ligand negative charge Is repelled by d electrons, ...
Design of molecular structure and synthetic approaches
... The molecular structures of these two new families of precursors (Fig. 5) demonstrate two structure types with analogous formal composition, MM′2X12, but different geometries: the linear chain one giving easier hydrolysis and less solution stability and the ring-type one with higher solution stabili ...
... The molecular structures of these two new families of precursors (Fig. 5) demonstrate two structure types with analogous formal composition, MM′2X12, but different geometries: the linear chain one giving easier hydrolysis and less solution stability and the ring-type one with higher solution stabili ...
Fulltext: english, pdf
... coordinated to different biomolecules and participating in many biochemical reactions where they play a crucial role. Carbohydrates, although involved in many biochemical processes, immunological events, and pathological conditions, exhibit relatively poor coordinating properties and form weak compl ...
... coordinated to different biomolecules and participating in many biochemical reactions where they play a crucial role. Carbohydrates, although involved in many biochemical processes, immunological events, and pathological conditions, exhibit relatively poor coordinating properties and form weak compl ...
Coordination Complexes Involving Electroactive
... cyanoethylthio)tetrathiafulvalene ligand L10 (Fig. 1) [76]. They consist of oxidized TTF derivatives covalently linked to a 3d or 4d (for Cd(II)) transition metal as a result of a metal– nitrile interaction, which yields 2D networks (Fig. 8). The centrosymmetric coordination spheres of the two metal ...
... cyanoethylthio)tetrathiafulvalene ligand L10 (Fig. 1) [76]. They consist of oxidized TTF derivatives covalently linked to a 3d or 4d (for Cd(II)) transition metal as a result of a metal– nitrile interaction, which yields 2D networks (Fig. 8). The centrosymmetric coordination spheres of the two metal ...
Reductive Elimination
... oxidation states because the formal oxidation state of the metal is reduced by two units in the reaction. ...
... oxidation states because the formal oxidation state of the metal is reduced by two units in the reaction. ...
A fluoro-bridged dinuclear nickel(II) compound from
... Fig. 1 UV-vis spectrum of complex (1) (curve 1) and the (L) (curve 2) in CH2Cl2. ...
... Fig. 1 UV-vis spectrum of complex (1) (curve 1) and the (L) (curve 2) in CH2Cl2. ...
Metal-Ligand Exchange Kinetics in Platinum and Ruthenium
... This overview will begin with a brief introduction to the molecular, kinetic and thermodynamic details of the coordination chemistry of medicinally relevant metals, focusing on platinum, ruthenium and other noble metals that have been shown to possess important biological properties. The metal–liga ...
... This overview will begin with a brief introduction to the molecular, kinetic and thermodynamic details of the coordination chemistry of medicinally relevant metals, focusing on platinum, ruthenium and other noble metals that have been shown to possess important biological properties. The metal–liga ...
Palladium Complexes Containing Potentially
... Synthesis of Complexes Previously reported syntheses of chelating pyridylidene complexes generally involve C–H bond activation strategies,[9b–9f] although the heteroatom assistance in this process may be different from that of a classical cyclometalation.[12] In this work, we have chosen an oxidativ ...
... Synthesis of Complexes Previously reported syntheses of chelating pyridylidene complexes generally involve C–H bond activation strategies,[9b–9f] although the heteroatom assistance in this process may be different from that of a classical cyclometalation.[12] In this work, we have chosen an oxidativ ...
Full-Text PDF
... structural features and potential to be applied as novel functional materials [1–7]. The final assembly can be influenced by numerous factors, such as geometric requirements of metal centers, shape and nature of the ligands, reaction routes, solvents, templates, pH of the reactive medium and counter ...
... structural features and potential to be applied as novel functional materials [1–7]. The final assembly can be influenced by numerous factors, such as geometric requirements of metal centers, shape and nature of the ligands, reaction routes, solvents, templates, pH of the reactive medium and counter ...
INTERPRETATION OF INFRARED SPECTRA Hydrocarbons
... Hydrocarbons show IR absorption peaks between 2800 and 3300 cm-1 due to C-H stretching vibrations. The hybridization of the carbon affects the exact position of the absorption — stiffer bonds vibrate at higher frequencies. sp3 C-H: 2800-3000, sp2 C-H: 3000-3100, sp C-H: 3300 cm-1. ...
... Hydrocarbons show IR absorption peaks between 2800 and 3300 cm-1 due to C-H stretching vibrations. The hybridization of the carbon affects the exact position of the absorption — stiffer bonds vibrate at higher frequencies. sp3 C-H: 2800-3000, sp2 C-H: 3000-3100, sp C-H: 3300 cm-1. ...
Photoredox Catalysis Unlocks Single
... challenging, requiring the use of sensitive reagents or harsh conditions. In this arena, limited stoichiometric precedent employing open-shell intermediates has been reported; as such, the simultaneous discovery by the Macmillan, Doyle, and Molander groups that photoredox catalysis can be used to en ...
... challenging, requiring the use of sensitive reagents or harsh conditions. In this arena, limited stoichiometric precedent employing open-shell intermediates has been reported; as such, the simultaneous discovery by the Macmillan, Doyle, and Molander groups that photoredox catalysis can be used to en ...
The Organogallium Subhalide R Ga I as Starting Compound for the
... liumdiiodide R(I)Ga-Ga(I)R 1 [R = C(SiMe 3 )3 ] in nhexane at room temperature gave a very slow reaction only. A complicated mixture was formed, which contained a considerable quantity of the starting compound 1 beside several products of unknown composition. Recrystallization from cyclopentane yiel ...
... liumdiiodide R(I)Ga-Ga(I)R 1 [R = C(SiMe 3 )3 ] in nhexane at room temperature gave a very slow reaction only. A complicated mixture was formed, which contained a considerable quantity of the starting compound 1 beside several products of unknown composition. Recrystallization from cyclopentane yiel ...
1 Introduction
... precious metals is carried out by means of lixiviation with aqua regia, which yields a first separation into a soluble part and an insoluble residue. The soluble metals are gold and the metals of the primary platinum group and the insoluble ones are silver and the secondary PGMs. The platinum group ...
... precious metals is carried out by means of lixiviation with aqua regia, which yields a first separation into a soluble part and an insoluble residue. The soluble metals are gold and the metals of the primary platinum group and the insoluble ones are silver and the secondary PGMs. The platinum group ...
Common aldehydes and ketones
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
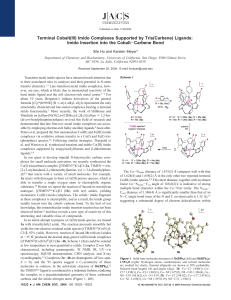
Terminal Cobalt(III) Imido Complexes Supported by Tris(Carbene
... the imido nitrogen (N8) and one of the coordinated carbene carbon (C3) ligands: the N8-C3 distance of 2.982 Å is considerably smaller than the sum of their van der Waals radii (3.25 Å).13 To gain insight into the electronic structure of complexes 3a4b, DFT calculations (ADF 2003.1, ZORA/TZP, BP86) w ...
... the imido nitrogen (N8) and one of the coordinated carbene carbon (C3) ligands: the N8-C3 distance of 2.982 Å is considerably smaller than the sum of their van der Waals radii (3.25 Å).13 To gain insight into the electronic structure of complexes 3a4b, DFT calculations (ADF 2003.1, ZORA/TZP, BP86) w ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.