Coordination Compounds Coordination Compounds
... compounds are called coordination compounds. The chemistry of coordination compounds is an important and challenging area of modern inorganic chemistry. New concepts of chemical bonding and molecular structure have provided insights into the functioning of vital components of biological systems. Chl ...
... compounds are called coordination compounds. The chemistry of coordination compounds is an important and challenging area of modern inorganic chemistry. New concepts of chemical bonding and molecular structure have provided insights into the functioning of vital components of biological systems. Chl ...
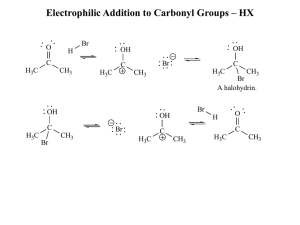
Electrophilic Addition to Carbonyl Groups – HX
... These compounds are v. strong nucleophiles – carbonyl does not necessarily need to be protonated prior to attack pH of 6-7 is required to make a good leaving group ...
... These compounds are v. strong nucleophiles – carbonyl does not necessarily need to be protonated prior to attack pH of 6-7 is required to make a good leaving group ...
Chapter 22s - Valdosta State University
... pigments: Fe4[Fe(CN)6)3 14 H2O (prussian blue), TiO2 (white). • Ions give color to gemstons: Iron(II) ions give yellow color in citrine and chromium(III) ions produce the red color of a ruby. Valdosta State University ...
... pigments: Fe4[Fe(CN)6)3 14 H2O (prussian blue), TiO2 (white). • Ions give color to gemstons: Iron(II) ions give yellow color in citrine and chromium(III) ions produce the red color of a ruby. Valdosta State University ...
Analytical Chemistry I lecture note
... product resulting from a reaction between a metal ion and a ligand is referred to as a coordination compound or complex ion. Central atom Ligands(unidentate) [CoCl(NH3)5]Cl2 Ionization sphere Coordination sphere Metal ions are Lewis acids, ligands are Lewis bases. Ag+ ...
... product resulting from a reaction between a metal ion and a ligand is referred to as a coordination compound or complex ion. Central atom Ligands(unidentate) [CoCl(NH3)5]Cl2 Ionization sphere Coordination sphere Metal ions are Lewis acids, ligands are Lewis bases. Ag+ ...
Copper perchlorate: Efficient acetylation catalyst
... phenols, amines and thiols are primary important functional group transformations in organic synthesis usually achieved by using acetic anhydride [1,2]. Several methods are available to protect the heteroatoms [3–21] and aldehydes [22–34] by using various metal salts e.g. chlorides, triflates, perch ...
... phenols, amines and thiols are primary important functional group transformations in organic synthesis usually achieved by using acetic anhydride [1,2]. Several methods are available to protect the heteroatoms [3–21] and aldehydes [22–34] by using various metal salts e.g. chlorides, triflates, perch ...
Class XII Chapter 9 – Coordination Compounds
... number, coordination polyhedron, homoleptic and heteroleptic. Answer (i) Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral mo ...
... number, coordination polyhedron, homoleptic and heteroleptic. Answer (i) Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral mo ...
RSC_NJ_C1NJ20460B 1..7
... complex stability order observed for metal catechol complexes.25 La(catechol)+ is the weakest (log Kf = 1.52 at pH 7.0) and Fe(catechol)+ (log Kf = 11.8 at pH 7.0) the most stable complex. The quenching efficiency of Fe, Sc, Al and Cu does not follow metal–catechol complex stability, suggesting that s ...
... complex stability order observed for metal catechol complexes.25 La(catechol)+ is the weakest (log Kf = 1.52 at pH 7.0) and Fe(catechol)+ (log Kf = 11.8 at pH 7.0) the most stable complex. The quenching efficiency of Fe, Sc, Al and Cu does not follow metal–catechol complex stability, suggesting that s ...
J. Am. Chem. Soc. 2009, 131, 7962
... rare cases have been observed in the solid state or in solution.10 Previous ROMP studies suggest that anti species may be orders of magnitude more reactive than syn species and that trans CdC bonds can form even though no anti alkylidene can be observed.5c Preventing formation of any significant amo ...
... rare cases have been observed in the solid state or in solution.10 Previous ROMP studies suggest that anti species may be orders of magnitude more reactive than syn species and that trans CdC bonds can form even though no anti alkylidene can be observed.5c Preventing formation of any significant amo ...
Amine-functionalized boehmite nanoparticle-supported
... The most promising property of these hydrothermally pro‐ cessed BNPs is the formation of a crystalline single‐phase product with no organic residue [44]. This was confirmed by the FT‐IR spectrum, PXRD pattern, and TG/DSC thermogram of this sample. In the FT‐IR spectrum ...
... The most promising property of these hydrothermally pro‐ cessed BNPs is the formation of a crystalline single‐phase product with no organic residue [44]. This was confirmed by the FT‐IR spectrum, PXRD pattern, and TG/DSC thermogram of this sample. In the FT‐IR spectrum ...
Coordination Chemistry Reviews Transition metal and nitrogen
... fused six-membered rings. The clusters representing (7,0) CNT and the (5,5) CNT contain 23 fused six-membered rings, respectively, as we show in Fig. 1(b) and (c). We have studied clusters containing Fe coordinated to two and four N atoms, denoted as Fe–2N and Fe–4N, respectively [65]. Three-nitroge ...
... fused six-membered rings. The clusters representing (7,0) CNT and the (5,5) CNT contain 23 fused six-membered rings, respectively, as we show in Fig. 1(b) and (c). We have studied clusters containing Fe coordinated to two and four N atoms, denoted as Fe–2N and Fe–4N, respectively [65]. Three-nitroge ...
The 16 and 18 Electron Rule in Organometallic Chemistry and
... joined. With less electronegative olefins, such as C,H4 or C2F4, it may be more convenient to regard their co-ordination as a Lewis base addition rather than as an oxidative addition. The real extent of electron transfer must be determined by ESCA or some other means. The count of metal valence elec ...
... joined. With less electronegative olefins, such as C,H4 or C2F4, it may be more convenient to regard their co-ordination as a Lewis base addition rather than as an oxidative addition. The real extent of electron transfer must be determined by ESCA or some other means. The count of metal valence elec ...
Syntheses, spectral characterization, thermal properties and DNA
... and ligands. i.e. one mole of metal salt reacted with two moles of 1,10-phenanthroline and one mole of ligands L1/L2 or L3 to give the corresponding metal complexes. The elemental analysis data for ligands and complexes is given in Table.1. All the compounds show the analytical results close to the ...
... and ligands. i.e. one mole of metal salt reacted with two moles of 1,10-phenanthroline and one mole of ligands L1/L2 or L3 to give the corresponding metal complexes. The elemental analysis data for ligands and complexes is given in Table.1. All the compounds show the analytical results close to the ...
Room temperature ionic liquid as a novel medium for liquid/liquid
... Room temperature ionic liquids (RTILs) have been used as novel solvents to replace traditional volatile organic solvents in organic synthesis, solvent extraction, and electrochemistry. The hydrophobic character and water immiscibility of certain ionic liquids allow their use in solvent extraction of ...
... Room temperature ionic liquids (RTILs) have been used as novel solvents to replace traditional volatile organic solvents in organic synthesis, solvent extraction, and electrochemistry. The hydrophobic character and water immiscibility of certain ionic liquids allow their use in solvent extraction of ...
Adsorption of cesium, thallium, strontium and cobalt radionuclides
... min.. However, in order to be sure that adsorption equilibrium between metallic ions and activated carbon was reached, 60 min. equilibration time was employed in all sequent measurements. It is important to note the small adsorption capacity of these carbon samples against Cs+ ; it may be due to the ...
... min.. However, in order to be sure that adsorption equilibrium between metallic ions and activated carbon was reached, 60 min. equilibration time was employed in all sequent measurements. It is important to note the small adsorption capacity of these carbon samples against Cs+ ; it may be due to the ...
Homoleptic Two-Coordinate Silylamido Complexes of Chromium(I
... room temperature for 2 and 8, respectively, are in good agreement with the Evans’ measurements. Compound 2 exhibits a steady decrease in susceptibility upon lowering the temperature, which indicates magnetic anisotropy and temperature-independent paramagnetism (Figure 5). A small field dependence at ...
... room temperature for 2 and 8, respectively, are in good agreement with the Evans’ measurements. Compound 2 exhibits a steady decrease in susceptibility upon lowering the temperature, which indicates magnetic anisotropy and temperature-independent paramagnetism (Figure 5). A small field dependence at ...
Dinuclear Nickel(II) and Palladium(II) Complexes in Combination
... During our work, we became aware that only singular examples of homodinuclear Ni II -, PdII -, CoII and CrIII -complexes [15, 18, 22, 24, 69] were investigated. Thus, we present here the first polymerization results of a series of homodinuclear nickel(II) and palladium(II) complexes 1 to 10 with Sch ...
... During our work, we became aware that only singular examples of homodinuclear Ni II -, PdII -, CoII and CrIII -complexes [15, 18, 22, 24, 69] were investigated. Thus, we present here the first polymerization results of a series of homodinuclear nickel(II) and palladium(II) complexes 1 to 10 with Sch ...
Hypervalent Compounds as Ligands
... most pronounced for M = Mn. In the case of “end-on” adducts, the situation with respect to electron densities is very similar (Table 2). The charge of It, the iodine connected to the metal center, is substantially reduced (−0.17 and −0.20 for Cr- and Mn-derivatives) in comparison with the charge in ...
... most pronounced for M = Mn. In the case of “end-on” adducts, the situation with respect to electron densities is very similar (Table 2). The charge of It, the iodine connected to the metal center, is substantially reduced (−0.17 and −0.20 for Cr- and Mn-derivatives) in comparison with the charge in ...
ON THE ROAD TO CARBENE AND CARBYNE COMPLEXES
... derivatives. In the second type, only the π electrons of the double bond are used for binding the organic molecule to the metal atom. In this way, we obtain π complexes (5,6) (Fig. 1b), the first representative of this being Zeise’s salt K[PtC1 3( C2H 4)], which was prepared as early as 1827 (7). Su ...
... derivatives. In the second type, only the π electrons of the double bond are used for binding the organic molecule to the metal atom. In this way, we obtain π complexes (5,6) (Fig. 1b), the first representative of this being Zeise’s salt K[PtC1 3( C2H 4)], which was prepared as early as 1827 (7). Su ...
File
... e) Metallic character increases on moving down the group. N and P are non metals, As and Sb are metalloids and Bi is a metal. On moving down the group, the atomic size and the screening effect of the intervening electrons increases. As a result the ionization enthalpy decreases. In other words, vale ...
... e) Metallic character increases on moving down the group. N and P are non metals, As and Sb are metalloids and Bi is a metal. On moving down the group, the atomic size and the screening effect of the intervening electrons increases. As a result the ionization enthalpy decreases. In other words, vale ...
nitrogen family study notes
... interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result, the catenation tendency is weaker in nitrogen. k) Nitrogen, due to the absence of d orbitals, cannot form d pi—p pi bonds as the heavier elements can, e.g., R3P=O, R3P=CH2. l) The reducing character ...
... interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result, the catenation tendency is weaker in nitrogen. k) Nitrogen, due to the absence of d orbitals, cannot form d pi—p pi bonds as the heavier elements can, e.g., R3P=O, R3P=CH2. l) The reducing character ...
Isocyanide Chemistry
... • The first isocyanide complex Ag(CNR)(CN) was obtained by Gautierin 1869. • But until 1977, the first zero-valent isocyanide complexes Co2(CNR)8 were obtained. • Now, a large number of isocyanide complexes involving metals from Group 5 to Group 11 and Ti. ...
... • The first isocyanide complex Ag(CNR)(CN) was obtained by Gautierin 1869. • But until 1977, the first zero-valent isocyanide complexes Co2(CNR)8 were obtained. • Now, a large number of isocyanide complexes involving metals from Group 5 to Group 11 and Ti. ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.