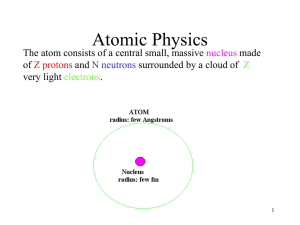
1. Atomic Structure
... (2) As long as the electron revolves in a particular orbit it can neither loose nor gain energy i.e energy of an electron in a particular orbit remains constant. Hence these orbits are called stationary orbits. ...
... (2) As long as the electron revolves in a particular orbit it can neither loose nor gain energy i.e energy of an electron in a particular orbit remains constant. Hence these orbits are called stationary orbits. ...
Periodic Properties of the Elements Effective Nuclear Charge, Zeff
... value of S. (Note: The electron does not screen itself.) 3. Electrons for which n is one less than n for the electron of interest contribute 0.85 to the value of S, while those with even smaller values of n contribute 1.00. ...
... value of S. (Note: The electron does not screen itself.) 3. Electrons for which n is one less than n for the electron of interest contribute 0.85 to the value of S, while those with even smaller values of n contribute 1.00. ...
Chapter 5
... The resolution is limited by the scattering of secondary electrons, that cause damage of the photoresist even at energies as low as a few eVs. Copyright Stuart Lindsay (2008) ...
... The resolution is limited by the scattering of secondary electrons, that cause damage of the photoresist even at energies as low as a few eVs. Copyright Stuart Lindsay (2008) ...
genchem study guide test_4a
... B Only a max of 2 electrons in each orbital and they must have opposite spins C Subdivision of energy level; the numeric value of energy level is equal to the total number of these in that energy level D Empty Bus Seat Rule; electrons occupy equal‐ energy orbitals so that a maximum number of u ...
... B Only a max of 2 electrons in each orbital and they must have opposite spins C Subdivision of energy level; the numeric value of energy level is equal to the total number of these in that energy level D Empty Bus Seat Rule; electrons occupy equal‐ energy orbitals so that a maximum number of u ...
1 - Optus
... Compare qualitatively the relative number of free electrons that can drift from atom to atom in conductors, semiconductors and insulators. Conductors contain high numbers of free electrons in the conduction band. Under normal conditions, insulators and semiconductors have far fewer free electrons th ...
... Compare qualitatively the relative number of free electrons that can drift from atom to atom in conductors, semiconductors and insulators. Conductors contain high numbers of free electrons in the conduction band. Under normal conditions, insulators and semiconductors have far fewer free electrons th ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
Energy Levels and Sub
... The difference between the classical meaning and the actual meaning of the quantum numbers is that in the classical meaning, scientists still thought of electrons as particles orbiting a nucleus in a defined path. Once Schroedinger showed that treating an electron as a wave resulted in calculations ...
... The difference between the classical meaning and the actual meaning of the quantum numbers is that in the classical meaning, scientists still thought of electrons as particles orbiting a nucleus in a defined path. Once Schroedinger showed that treating an electron as a wave resulted in calculations ...
Electron Spin I - Rutgers Physics
... • The physics of quantum mechanics is completely given by the postulates of the previous lecture. Everything else is built on them. • We will now give a concrete example of the use of these postulates for the simplest nontrivial system possible, a system who’s states are elements of a 2-dimensional ...
... • The physics of quantum mechanics is completely given by the postulates of the previous lecture. Everything else is built on them. • We will now give a concrete example of the use of these postulates for the simplest nontrivial system possible, a system who’s states are elements of a 2-dimensional ...
m L
... • As you know, the d-orbitals hold a max of 10 electrons • These d-orbitals, when possible, will assume a half-filled, or fully-filled configuration by taking an electron from the ns orbital • This occurs when a transition metal has 4 or 9 valence d electrons Example: Cr [Ar] 4s2 3d4 ...
... • As you know, the d-orbitals hold a max of 10 electrons • These d-orbitals, when possible, will assume a half-filled, or fully-filled configuration by taking an electron from the ns orbital • This occurs when a transition metal has 4 or 9 valence d electrons Example: Cr [Ar] 4s2 3d4 ...
Microscopy with Electron Diffraction Overview
... • Since the diffraction zone is shallow, the specimen surface must no be obscured by: ...
... • Since the diffraction zone is shallow, the specimen surface must no be obscured by: ...
A Student Introduction to Solar Energy
... be clear. All the aspects presented in this chapter will be discussed in greater detail in the following chapters. The working principle of solar cells is based on the photovoltaic effect, i.e. the generation of a potential difference at the junction of two different materials in response to electro ...
... be clear. All the aspects presented in this chapter will be discussed in greater detail in the following chapters. The working principle of solar cells is based on the photovoltaic effect, i.e. the generation of a potential difference at the junction of two different materials in response to electro ...
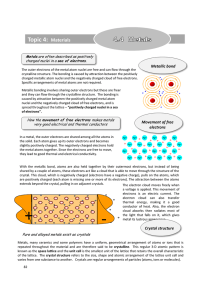
Topic 4: Materials - Education Umbrella
... With the metallic bond, atoms are also held together by their outermost electrons, but instead of being shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), ...
... With the metallic bond, atoms are also held together by their outermost electrons, but instead of being shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), ...
Electron-beam lithography

Electron-beam lithography (often abbreviated as e-beam lithography) is the practice of scanning a focused beam of electrons to draw custom shapes on a surface covered with an electron-sensitive film called a resist (""exposing""). The electron beam changes the solubility of the resist, enabling selective removal of either the exposed or non-exposed regions of the resist by immersing it in a solvent (""developing""). The purpose, as with photolithography, is to create very small structures in the resist that can subsequently be transferred to the substrate material, often by etching.The primary advantage of electron-beam lithography is that it can draw custom patterns (direct-write) with sub-10 nm resolution. This form of maskless lithography has high resolution and low throughput, limiting its usage to photomask fabrication, low-volume production of semiconductor devices, and research & development.