Chemistry Test Ch 11 Stoichiometry
... Directions: Show all work!!! No Work Shown, no credit given! Include all units and chemical formulas. 1. Balance the following equation and work the problems below: ____N2 + ___ H2 ___ NH3 A. If 6.52 L of H2 react with excess nitrogen what volume of NH3 is produced? B. In order to produce 6.52 L o ...
... Directions: Show all work!!! No Work Shown, no credit given! Include all units and chemical formulas. 1. Balance the following equation and work the problems below: ____N2 + ___ H2 ___ NH3 A. If 6.52 L of H2 react with excess nitrogen what volume of NH3 is produced? B. In order to produce 6.52 L o ...
Photoactivation mechanism of PAmCherry based on crystal
... PAmCherry1, was reported, which complements the photoactivatable GFP by providing a red super-resolution color. PAmCherry1 is originally ‘‘dark’’ but exhibits red fluorescence after UV-violet light irradiation. To define the structural basis of PAmCherry1 photoactivation, we determined its crystal s ...
... PAmCherry1, was reported, which complements the photoactivatable GFP by providing a red super-resolution color. PAmCherry1 is originally ‘‘dark’’ but exhibits red fluorescence after UV-violet light irradiation. To define the structural basis of PAmCherry1 photoactivation, we determined its crystal s ...
Document
... of chemical potential energy. This stored energy is related to the relative positions of particles and the strengths of the bonds between them. Potential energy is stored or released as the positions of the particles change, just as it is when a spring is stretched and then released. As bonds break ...
... of chemical potential energy. This stored energy is related to the relative positions of particles and the strengths of the bonds between them. Potential energy is stored or released as the positions of the particles change, just as it is when a spring is stretched and then released. As bonds break ...
Section 4.9 Oxidation–Reduction Reactions
... consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product actually produced • Percent Yield – (actual/theor ...
... consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product actually produced • Percent Yield – (actual/theor ...
4 Expressing and Measuring Chemical Change
... much easier if you follow a good plan of attack. Keep the following hints in mind when attempting to balance an equation: • Begin by balancing atoms that appear in only one place on each side of the equation. Atoms that appear in too many places will be more difficult to balance and should always be ...
... much easier if you follow a good plan of attack. Keep the following hints in mind when attempting to balance an equation: • Begin by balancing atoms that appear in only one place on each side of the equation. Atoms that appear in too many places will be more difficult to balance and should always be ...
Press here to hemy 102 lab manual
... Every chemical change is accompanied by a change in energy, usually in the form of heat. The energy change of a reaction that occurs at constant pressure is termed the heat of reaction or the enthalpy change. The symbol ΔH is used to denote the enthalpy change. If heat is evolved, the reaction is ex ...
... Every chemical change is accompanied by a change in energy, usually in the form of heat. The energy change of a reaction that occurs at constant pressure is termed the heat of reaction or the enthalpy change. The symbol ΔH is used to denote the enthalpy change. If heat is evolved, the reaction is ex ...
(General Equilibrium) Part 1
... 3. Concentrations of pure solids or pure liquids are _________ (their activity is set to “1”.) when writing the equilibrium equation for any heterogeneous equilibrium. -The value for the concentrations of solids and liquids are incorporated into the value of Kc. C. Units for Kc – Kc is expressed wit ...
... 3. Concentrations of pure solids or pure liquids are _________ (their activity is set to “1”.) when writing the equilibrium equation for any heterogeneous equilibrium. -The value for the concentrations of solids and liquids are incorporated into the value of Kc. C. Units for Kc – Kc is expressed wit ...
CH 17 Study Guide with answer Key
... aqueous solutions are mixed together. First, calculate the concentrations of the ions in the final solution. Use the solubility product constant expression to calculate the ion product (Qsp ) for the substance that might precipitate. Compare the result with the Ksp of the substance. 7. What can you ...
... aqueous solutions are mixed together. First, calculate the concentrations of the ions in the final solution. Use the solubility product constant expression to calculate the ion product (Qsp ) for the substance that might precipitate. Compare the result with the Ksp of the substance. 7. What can you ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Acids and Bases in Solution - acids ionize in water to form H+ ions. More precisely, the H from the acid molecule is donated to a water molecule to form hydronium ion, H3O+. Most chemists use H+ and H3O+ interchangeably. - bases dissociate in water to form OH ions. Bases, like NH3, that do not ...
... Acids and Bases in Solution - acids ionize in water to form H+ ions. More precisely, the H from the acid molecule is donated to a water molecule to form hydronium ion, H3O+. Most chemists use H+ and H3O+ interchangeably. - bases dissociate in water to form OH ions. Bases, like NH3, that do not ...
111 Exam III OUTLINE TRO 1-3-11
... III. Lewis Acid-Base Concept A. DEFINITION Lewis Acid ⇨ A substance that is an electron pair acceptor (A covalent bond is made) ex. ...
... III. Lewis Acid-Base Concept A. DEFINITION Lewis Acid ⇨ A substance that is an electron pair acceptor (A covalent bond is made) ex. ...
St. Xavier`s College – Autonomous Mumbai Syllabus for 3 Semester
... haloarenes, phenols, ethers and epoxides and understand mechanisms of certain reactions. 5. To introduce the aspects of a chemical plant and study the sources and classification of fuels. ...
... haloarenes, phenols, ethers and epoxides and understand mechanisms of certain reactions. 5. To introduce the aspects of a chemical plant and study the sources and classification of fuels. ...
4_ Chemical reactions
... Some examples are shown below: 2Mg(s) + O2(g) → 2MgO(s) 2Na(s) + Cl2(g) → 2NaCl(s) SO3(g) + H2O(l) → H2SO4(aq) II) Decomposition Reactions In a decomposition reaction, a reactant splits into two or more simpler products. The general form of the reaction is (AB → A + B). Some examples are shown below ...
... Some examples are shown below: 2Mg(s) + O2(g) → 2MgO(s) 2Na(s) + Cl2(g) → 2NaCl(s) SO3(g) + H2O(l) → H2SO4(aq) II) Decomposition Reactions In a decomposition reaction, a reactant splits into two or more simpler products. The general form of the reaction is (AB → A + B). Some examples are shown below ...
advanced chemistry may 2011 marking scheme
... (b) Carbon monoxide and water vapour, each at 0.5 atm, were introduced into a reaction vessel and allowed to reach equilibrium at 2000 K. Calculate (i) the partial pressure of each gas in the vessel at equilibrium If the partial pressure of CO2 at equilibrium = x atm, then p. press. of H2 = x atm an ...
... (b) Carbon monoxide and water vapour, each at 0.5 atm, were introduced into a reaction vessel and allowed to reach equilibrium at 2000 K. Calculate (i) the partial pressure of each gas in the vessel at equilibrium If the partial pressure of CO2 at equilibrium = x atm, then p. press. of H2 = x atm an ...
Topic 14 - Fertilisers
... However, nitrogen is un-reactive but not inert. This means it is difficult getting it to react but it can be done (using electricity i.e. lightening or a spark plug). The nitrogen will form oxides which dissolve in water forming acids. Nitrogen dioxide, a brown gas, can be made when air (21% oxygen ...
... However, nitrogen is un-reactive but not inert. This means it is difficult getting it to react but it can be done (using electricity i.e. lightening or a spark plug). The nitrogen will form oxides which dissolve in water forming acids. Nitrogen dioxide, a brown gas, can be made when air (21% oxygen ...
Chapter 4
... Vinegar is an aqueous solution of acetic acid, HC2H3O2, which is often written as HAc for simplicity. A 12.5 mL sample of vinegar was titrated with a 0.504 M solution of NaOH. The titration required 15.40 mL of the base solution in order to reach the endpoint. What is the molar concentration of HAc ...
... Vinegar is an aqueous solution of acetic acid, HC2H3O2, which is often written as HAc for simplicity. A 12.5 mL sample of vinegar was titrated with a 0.504 M solution of NaOH. The titration required 15.40 mL of the base solution in order to reach the endpoint. What is the molar concentration of HAc ...
National 5 - Deans Community High School
... When the packet is rubbed between the hands, the inner bag breaks open and its contents mix with the crystals. The packet can then be placed in a pocket or glove to keep the hands warm for a period of time. If the packet is opened after being used it is found to contain only a black powder. Suggest ...
... When the packet is rubbed between the hands, the inner bag breaks open and its contents mix with the crystals. The packet can then be placed in a pocket or glove to keep the hands warm for a period of time. If the packet is opened after being used it is found to contain only a black powder. Suggest ...
PDF File
... concentration dependences in which the maximal rate constant for reaction varied by more than 10-fold, which was accomplished by a 2′-H substitution at position -1 and by varying the pH (38). The affinity of S or P for the ribozyme is very high (see Figure 2B and Results), such that nonspecific loss ...
... concentration dependences in which the maximal rate constant for reaction varied by more than 10-fold, which was accomplished by a 2′-H substitution at position -1 and by varying the pH (38). The affinity of S or P for the ribozyme is very high (see Figure 2B and Results), such that nonspecific loss ...
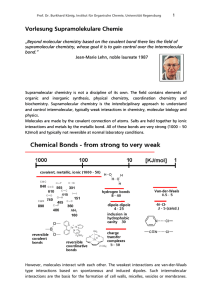
Vorlesung Supramolekulare Chemie
... of the energetic balance of the binding event and most of the binding enthalpy must be used to compensate the entropic energy loss. Receptor molecule or guest binding site for intermolecular molecular recognition must be preorganized. The conformation and orientation of functional groups in the non- ...
... of the energetic balance of the binding event and most of the binding enthalpy must be used to compensate the entropic energy loss. Receptor molecule or guest binding site for intermolecular molecular recognition must be preorganized. The conformation and orientation of functional groups in the non- ...
Aluminum and Copper
... Possible signs of a chemical reaction include color changes, temperature changes, formation of a precipitate, and gas evolution. The reaction of aluminum and copper(II) chloride is very vigorous—the reaction mixture gets very hot as heat is released, the blue color due to the Cu(II) ions fades, t ...
... Possible signs of a chemical reaction include color changes, temperature changes, formation of a precipitate, and gas evolution. The reaction of aluminum and copper(II) chloride is very vigorous—the reaction mixture gets very hot as heat is released, the blue color due to the Cu(II) ions fades, t ...
Oxidation-Reduction Reactions
... able to keep track of electron transfer when new compounds are formed. There are several general rules that are followed when assigning oxidation numbers to atoms: For an atom in elemental form (an element standing alone with no charge) the oxidation number is always zero. Examples: ...
... able to keep track of electron transfer when new compounds are formed. There are several general rules that are followed when assigning oxidation numbers to atoms: For an atom in elemental form (an element standing alone with no charge) the oxidation number is always zero. Examples: ...
by John Mu
... Identify polyethylene as an addition polymer and explain the meaning of this term The double bond opens up to form two single bonds that can bond with other things, this is called an addition reaction If these connect to other similar molecules (monomers), it is an addition polymer ...
... Identify polyethylene as an addition polymer and explain the meaning of this term The double bond opens up to form two single bonds that can bond with other things, this is called an addition reaction If these connect to other similar molecules (monomers), it is an addition polymer ...
ď - Google Sites
... 1. Count the total valence electrons in the entity by adding the valence electrons of each atom. If the entity is a polyatomic ion, add (usually) or subtract valence electrons to account for the net charge, one for each unit of charge. 2. Arrange the peripheral atom symbols around the central atom s ...
... 1. Count the total valence electrons in the entity by adding the valence electrons of each atom. If the entity is a polyatomic ion, add (usually) or subtract valence electrons to account for the net charge, one for each unit of charge. 2. Arrange the peripheral atom symbols around the central atom s ...
Synthesis of monoselenanedisulfanediphosphonate by the reaction
... (10 mmol) of ascorbic acid in 75 ml of water; then a solution of potassium dihydrogenmonothiophosphate, KH2PO3S, prepared by dissolution of 3.04 g (20 mmol) of KH2PO3S in 75 ml of water was added into it. The solution (2) was slowly added to the solution (1), and a clear yellowish-green solution of ...
... (10 mmol) of ascorbic acid in 75 ml of water; then a solution of potassium dihydrogenmonothiophosphate, KH2PO3S, prepared by dissolution of 3.04 g (20 mmol) of KH2PO3S in 75 ml of water was added into it. The solution (2) was slowly added to the solution (1), and a clear yellowish-green solution of ...
Unit 5 2 Thermodynamics Enthalpy
... That is: When a reaction is the sum of two or more other reactions, the ΔH for the overall process is the sum of the enthalpy changes for the constituent reactants 1) Hess’s Law is a neat means of calculating enthalpy changes which may be fleeting or difficult to measure. a) per your text (p187) “It ...
... That is: When a reaction is the sum of two or more other reactions, the ΔH for the overall process is the sum of the enthalpy changes for the constituent reactants 1) Hess’s Law is a neat means of calculating enthalpy changes which may be fleeting or difficult to measure. a) per your text (p187) “It ...
3: Haloalkanes, Alcohols, Ethers, and Amines
... (3.9) is much greater than that of C (2.5). In contrast, C-H bonds are not very polar because the electronegativities of H (2.3) and C (2.5) are about the same. Positive (+) values for the electronegativity differences in Table 3.1 mean that C is positively polarized (δ+) while its attached atom is ...
... (3.9) is much greater than that of C (2.5). In contrast, C-H bonds are not very polar because the electronegativities of H (2.3) and C (2.5) are about the same. Positive (+) values for the electronegativity differences in Table 3.1 mean that C is positively polarized (δ+) while its attached atom is ...