Chemical Reactions
... General Reaction Equations Remember that there are two possible types of combustion reactions. •Complete combustion reactions yield carbon dioxide and water. •Incomplete combustion reactions yield carbon monoxide and water. ...
... General Reaction Equations Remember that there are two possible types of combustion reactions. •Complete combustion reactions yield carbon dioxide and water. •Incomplete combustion reactions yield carbon monoxide and water. ...
- International Journal of Multidisciplinary Research and
... new method for hydrogen production from methane steam reforming by the recovery of biogas using tri-ethanol amine absorption/stripping is the main purpose of the study. Hydrogen produced is then supplied to a PEM fuel cell to produce electricity. Throughout the whole study, different types of public ...
... new method for hydrogen production from methane steam reforming by the recovery of biogas using tri-ethanol amine absorption/stripping is the main purpose of the study. Hydrogen produced is then supplied to a PEM fuel cell to produce electricity. Throughout the whole study, different types of public ...
final exam review packet
... A. The solution’s ___________________ point is higher. (when you cook pasta) B. The solution’s ___________________ point is lower. (when you add salt to roads in winter) C. These properties are called __________________properties because they depend on the concentration of solute but not the nature ...
... A. The solution’s ___________________ point is higher. (when you cook pasta) B. The solution’s ___________________ point is lower. (when you add salt to roads in winter) C. These properties are called __________________properties because they depend on the concentration of solute but not the nature ...
Chemistry 123: Physical and Organic Chemistry
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
Welcome to AP Chemistry!
... 1. Purchase your own copy of 5 Steps to a 5 on the AP: Chemistry, John T Moore, McGraw Hill. (You can purchase them at Amazon.com Online, probably the more recent the publication the most current information however any version will work.) 2. Buy a few color highlighters. 3. Read and study thru the ...
... 1. Purchase your own copy of 5 Steps to a 5 on the AP: Chemistry, John T Moore, McGraw Hill. (You can purchase them at Amazon.com Online, probably the more recent the publication the most current information however any version will work.) 2. Buy a few color highlighters. 3. Read and study thru the ...
SAMPLE QUESTION PAPER CHEMISTRY (043) CLASS XII (2013-14)
... 25. (a) Aniline is less basic than ammonia because due to resonance , lone pair on N get involved and is not available for donation but in ammonia it is not so. Lone pair of electron ...
... 25. (a) Aniline is less basic than ammonia because due to resonance , lone pair on N get involved and is not available for donation but in ammonia it is not so. Lone pair of electron ...
Chemistry IGCSE Revision PDF File
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
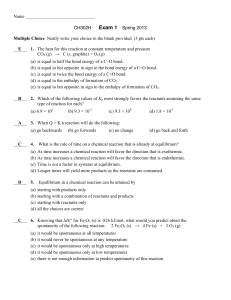
Exam 1 Key
... it would be spontaneous only at high temperatures it would be spontaneous only at low temperatures there is not enough information to predict spontaneity of this reaction ...
... it would be spontaneous only at high temperatures it would be spontaneous only at low temperatures there is not enough information to predict spontaneity of this reaction ...
Chapter 2: Chemical Reactions Section 1
... Chemical Reactions Section 1: Observing Chemical Changes How can changes in matter be described? In terms of two kinds of propertiesphysical properties and chemical properties Changes in matter can be described in terms of physical or chemical changes ...
... Chemical Reactions Section 1: Observing Chemical Changes How can changes in matter be described? In terms of two kinds of propertiesphysical properties and chemical properties Changes in matter can be described in terms of physical or chemical changes ...
File
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
... 25 CH3CH2COCH2CH3 reacts with hydrogen cyanide to form an organic product called a cyanohydrin. Which feature applies to the cyanohydrin product? A ...
Dr. Baxley`s Thermodynamics Worksheet
... Calculate the maximum work that could be obtained at 25 °C and 1 atm by burning 1 mol of liquid ethanol. You will need to write and balance the chemical equation. Remember that when a hydrocarbon burns, it reacts with oxygen to produce carbon dioxide and gaseous ...
... Calculate the maximum work that could be obtained at 25 °C and 1 atm by burning 1 mol of liquid ethanol. You will need to write and balance the chemical equation. Remember that when a hydrocarbon burns, it reacts with oxygen to produce carbon dioxide and gaseous ...
FXM Rev 1 Key - Grande Cache Community High School
... or melted into a liquid form. Acids, bases and salts are examples. cation This is a positive ion that has form after it lost one or more electrons. Ca2+ is an example. intermolecular force This is a force of attractions between two different molecules. Hydrogen bonding is an example. chemical reacti ...
... or melted into a liquid form. Acids, bases and salts are examples. cation This is a positive ion that has form after it lost one or more electrons. Ca2+ is an example. intermolecular force This is a force of attractions between two different molecules. Hydrogen bonding is an example. chemical reacti ...
So where did all the matter on Earth come from - Bennatti
... letters. The first letter is always capitalized. If it has two or three letters only the first letter is capitalized. For example the chemical symbol for the element magnesium is Mg. Note the letter g is lower case. This is important as Co is the element cobalt but CO is the compound carbon monoxide ...
... letters. The first letter is always capitalized. If it has two or three letters only the first letter is capitalized. For example the chemical symbol for the element magnesium is Mg. Note the letter g is lower case. This is important as Co is the element cobalt but CO is the compound carbon monoxide ...
File - Mr. Holz`s Website
... showed Sodium, Lithium, Ceasium, etc. being dropped in water? Hint: It has something to do with the valence electrons Know the difference between ionic and covalent bonding. a. In ionic bonding, when atoms gain or lose electrons, how does that change their overall charge? Also know that the resultin ...
... showed Sodium, Lithium, Ceasium, etc. being dropped in water? Hint: It has something to do with the valence electrons Know the difference between ionic and covalent bonding. a. In ionic bonding, when atoms gain or lose electrons, how does that change their overall charge? Also know that the resultin ...
Reactive Materials - NC State University
... Reactive liquids are chemicals that react vigorously with moisture or oxygen or other substances. Reactive solids are chemicals that react vigorously with moisture and other substances. The most common reactive solids include sodium, potassium and lithium metals, acid anhydrides and acid chlorides. ...
... Reactive liquids are chemicals that react vigorously with moisture or oxygen or other substances. Reactive solids are chemicals that react vigorously with moisture and other substances. The most common reactive solids include sodium, potassium and lithium metals, acid anhydrides and acid chlorides. ...
Balanced Chemical Equation
... • This can be put in your binder. It is not due until the 4th marking period. Answer the questions as we cover the subjects and hold onto it. (-5 points each time you ask for a new one.) • This simple reaction that you will do will summarize most of the things you will learn over the next few months ...
... • This can be put in your binder. It is not due until the 4th marking period. Answer the questions as we cover the subjects and hold onto it. (-5 points each time you ask for a new one.) • This simple reaction that you will do will summarize most of the things you will learn over the next few months ...
chemical reactions
... nitrogen gas, water vapor and powdered chromium (III) oxide. It looks like a volcano with ash being spread all over the place. ...
... nitrogen gas, water vapor and powdered chromium (III) oxide. It looks like a volcano with ash being spread all over the place. ...
Chemical Equations & Reactions
... Using Bond Energy Table, determine ∆H. ∆H = -482 kJ (482 kJ released = exothermic) ...
... Using Bond Energy Table, determine ∆H. ∆H = -482 kJ (482 kJ released = exothermic) ...
File
... Nitrogen dioxide + water(l) nitric acid + nitrogen monoxide Nitrogen dioxide(g) + water(l) nitric acid(aq) + nitrogen monoxide(g) NO2(g) + H2O(l) HNO3(aq) + NO(g) 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) ...
... Nitrogen dioxide + water(l) nitric acid + nitrogen monoxide Nitrogen dioxide(g) + water(l) nitric acid(aq) + nitrogen monoxide(g) NO2(g) + H2O(l) HNO3(aq) + NO(g) 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) ...
Class Notes 2
... Repeating values of Ф and Ψ along the main chain result in regular structure – repeating values of Ф =-57o and Ψ =-47o give a right-handed helical fold (α-helix) – repetitive values of Ф[-110,-140] and Ψ[+110,+135] give sub chains with conformations that allow interactions between nearby parallel ...
... Repeating values of Ф and Ψ along the main chain result in regular structure – repeating values of Ф =-57o and Ψ =-47o give a right-handed helical fold (α-helix) – repetitive values of Ф[-110,-140] and Ψ[+110,+135] give sub chains with conformations that allow interactions between nearby parallel ...
ppt - ChemConnections
... Examine the image above and select the correct statement below. A)NaHCO3 is the limiting reagent in the yellow and blue. B)HCl is definitely not the limiting reagent in all three cases. C)Equal numbers of moles of HCl and NaHCO3 may have reacted ...
... Examine the image above and select the correct statement below. A)NaHCO3 is the limiting reagent in the yellow and blue. B)HCl is definitely not the limiting reagent in all three cases. C)Equal numbers of moles of HCl and NaHCO3 may have reacted ...
IGCSE Revision document
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
... Sodium chloride NaCl is a ____________. There are __________ bonds between the two the same/ different numbers of electrons. elements _________ and _________. When these atoms bond one ____________ from the • Isotopes are atoms of the same element with ___________ atom is donated to the ____________ ...
Chemical reactions alter arrangements of atoms.
... A change in the state of a substance is an example of a physical change. The substance may have some different properties after a physical change, but it is still the same substance. For example, you know that water can exist in three different physical states: the solid state (ice), the liquid stat ...
... A change in the state of a substance is an example of a physical change. The substance may have some different properties after a physical change, but it is still the same substance. For example, you know that water can exist in three different physical states: the solid state (ice), the liquid stat ...
CHEMISTRY I Final..#1..rev 4KEY
... 26. A sample of a gas is contained in a closed rigid cylinder. According to kinetic molecular theory, what occurs when the gas inside the cylinder is heated? a. The number of gas molecules increases. b. The number of collisions between gas molecules per unit time decreases. c. The average velocity o ...
... 26. A sample of a gas is contained in a closed rigid cylinder. According to kinetic molecular theory, what occurs when the gas inside the cylinder is heated? a. The number of gas molecules increases. b. The number of collisions between gas molecules per unit time decreases. c. The average velocity o ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... commercial production of H2O2 is mainly based on a multistep process involving cyclic hydrogenation and oxidation of an alkyl anthraquinone in a complex working solution.2 This high-energy consuming process often has disadvantages, such as the cost of the quinone solvent system and the elaborate tre ...
... commercial production of H2O2 is mainly based on a multistep process involving cyclic hydrogenation and oxidation of an alkyl anthraquinone in a complex working solution.2 This high-energy consuming process often has disadvantages, such as the cost of the quinone solvent system and the elaborate tre ...