Glucose Support Formula
... cells of the pancreas.* • Alpha lipoic acid, a versatile nutrient that provides powerful antioxidant activity and sustains healthy glucose function.* • Panax ginseng and Eleutherococcus senticosus, or eleuthero, adaptogens that promote healthy glucose balance.* • Maitake mushroom extract, playing ...
... cells of the pancreas.* • Alpha lipoic acid, a versatile nutrient that provides powerful antioxidant activity and sustains healthy glucose function.* • Panax ginseng and Eleutherococcus senticosus, or eleuthero, adaptogens that promote healthy glucose balance.* • Maitake mushroom extract, playing ...
2.4 Chemical Reactions and Enzymes
... • Animals get their energy when they consume plants or other animals. • Humans release the energy needed to grow tall, to breathe, digest food, & etc. ...
... • Animals get their energy when they consume plants or other animals. • Humans release the energy needed to grow tall, to breathe, digest food, & etc. ...
Preview Sample 2 - Test Bank, Manual Solution, Solution Manual
... a. Even if students have had an introductory chemistry course in the past, it is not unusual for students to struggle with chemical notation. Surprisingly, many students will draw a water (H2O) molecule with two oxygen atoms and one hydrogen atom, with the idea that the “2” refers to “two O’s.” You ...
... a. Even if students have had an introductory chemistry course in the past, it is not unusual for students to struggle with chemical notation. Surprisingly, many students will draw a water (H2O) molecule with two oxygen atoms and one hydrogen atom, with the idea that the “2” refers to “two O’s.” You ...
Respiration and Photosynthesis
... • 6 carbon dioxide molecules eneter cycle from atmosphere and combine with 6 5 carbon molecules to make 12 3 carbon molecules • Energy from ATP and NADPH is used to convert the 12 3 carbon molecules into high energy forms • 2 of the 12 3 carbon molecules are removed from the cycle to be used by the ...
... • 6 carbon dioxide molecules eneter cycle from atmosphere and combine with 6 5 carbon molecules to make 12 3 carbon molecules • Energy from ATP and NADPH is used to convert the 12 3 carbon molecules into high energy forms • 2 of the 12 3 carbon molecules are removed from the cycle to be used by the ...
File
... Lactic acid is a chemical structure made out of carbon, hydrogen, and oxygen. It is also known as milk acid. Lactate is produced in the body during a chemical reaction, but lactic acid doesn’t form under such simple conditions. During hard exercise when anaerobic respiration occurs with aerobic resp ...
... Lactic acid is a chemical structure made out of carbon, hydrogen, and oxygen. It is also known as milk acid. Lactate is produced in the body during a chemical reaction, but lactic acid doesn’t form under such simple conditions. During hard exercise when anaerobic respiration occurs with aerobic resp ...
Biochemical and molecular-genetic methods of the study of
... atmosphere is a prerequisite of life on the Earth. Within the last decade the detailed structure of Photosystem II and the roles of its key subunits have been described. We have also fundamental knowledge of the principles of PSII biogenesis, i.e. de novo synthesis of PSII in the course of life and ...
... atmosphere is a prerequisite of life on the Earth. Within the last decade the detailed structure of Photosystem II and the roles of its key subunits have been described. We have also fundamental knowledge of the principles of PSII biogenesis, i.e. de novo synthesis of PSII in the course of life and ...
DEPARTMENT OF MICROBIOLOGY University of Delhi South campus New Delhi-110021 PhD Course work
... W. Whitaker & S.J. Hall, Elsevier India Pvt. Ltd. New Delhi-110001. Bioprocess Engineering Principles (2nd Edition, 2012) by Academic Press/Elsevier India Pvt. Ltd. New Delhi-110001. Bioprocess Engineering: Basic Concepts (2nd Edition, 2011) by Michael L. Shuler and FikertKargiPrentice Hall India le ...
... W. Whitaker & S.J. Hall, Elsevier India Pvt. Ltd. New Delhi-110001. Bioprocess Engineering Principles (2nd Edition, 2012) by Academic Press/Elsevier India Pvt. Ltd. New Delhi-110001. Bioprocess Engineering: Basic Concepts (2nd Edition, 2011) by Michael L. Shuler and FikertKargiPrentice Hall India le ...
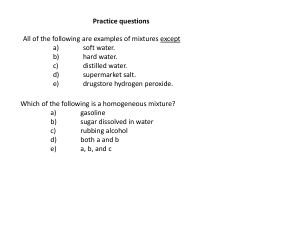
Practice questions
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
BIOL 1406 Discussion Questions: Photosynthesis
... 11. How are cyclic and noncyclic photophosphorylation similar? How are they different? 12. Explain why many plant species sometimes switch from noncyclic to cyclic photophosphorylation. 13. Describe the process of carbon fixation during the Calvin cycle. 14. During the Calvin cycle, how is G3P manuf ...
... 11. How are cyclic and noncyclic photophosphorylation similar? How are they different? 12. Explain why many plant species sometimes switch from noncyclic to cyclic photophosphorylation. 13. Describe the process of carbon fixation during the Calvin cycle. 14. During the Calvin cycle, how is G3P manuf ...
AMINOACID METABOLISM
... * This reaction is important as it reversibly links glutamate metabolism with TCA CYCLE through α Ketoglutarate. GDH – regulated allosterically – GTP & ATP inhibits & viceversa ...
... * This reaction is important as it reversibly links glutamate metabolism with TCA CYCLE through α Ketoglutarate. GDH – regulated allosterically – GTP & ATP inhibits & viceversa ...
DNA Review
... o the enzyme DNA polymerase attaches free-floating nucleotides in the nucleus to the parent strand to create the daughter strand ...
... o the enzyme DNA polymerase attaches free-floating nucleotides in the nucleus to the parent strand to create the daughter strand ...
File
... Alcohol molecules are covalent by nature. However, they contain the strongly polar O-H bond. Alcohol structure consists of alcohol molecules held together by a combination of weak Van-der-Waals forces and stronger hydrogen bonds. This has significant effects on the physical properties of alcohols. B ...
... Alcohol molecules are covalent by nature. However, they contain the strongly polar O-H bond. Alcohol structure consists of alcohol molecules held together by a combination of weak Van-der-Waals forces and stronger hydrogen bonds. This has significant effects on the physical properties of alcohols. B ...
Biuret test - WordPress.com
... amino acids form hydrogen bonds with surrounding water molecules (solvation layer). When proteins are present in salt solutions (e.g. ammonium sulfate), some of the water molecules in the solvation layer are attracted by salt ions. When salt concentration gradually increases, the number of water mol ...
... amino acids form hydrogen bonds with surrounding water molecules (solvation layer). When proteins are present in salt solutions (e.g. ammonium sulfate), some of the water molecules in the solvation layer are attracted by salt ions. When salt concentration gradually increases, the number of water mol ...
Slide 1
... – Occurs in animals – Starting molecules: 2 pyruvates and NADH (from glycolysis) – Produces: lactic acid – Lactic acid fermentation by microorganisms plays an essential role in the manufacturing of food products such as yogurt and cheese. ...
... – Occurs in animals – Starting molecules: 2 pyruvates and NADH (from glycolysis) – Produces: lactic acid – Lactic acid fermentation by microorganisms plays an essential role in the manufacturing of food products such as yogurt and cheese. ...
Sample Questions for Exam One Multiple Choice. Choose the
... b. atoms involved in a bond have an extreme difference in electronegativity c. valence shell electrons are equally shared d. valence shell electrons are transferred from one atom to another e. positively charged ions are formed 11. Examine the chemical C6H12O6, Which of the following statements abou ...
... b. atoms involved in a bond have an extreme difference in electronegativity c. valence shell electrons are equally shared d. valence shell electrons are transferred from one atom to another e. positively charged ions are formed 11. Examine the chemical C6H12O6, Which of the following statements abou ...
Amino acid Catabolism
... the synthesis of urea, which is quantitatively the most important route for disposing of nitrogen from the body. • In the second phase of amino acid catabolism, the carbon skeletons of the α-ketoacids are converted to common intermediates of energy producing, metabolic pathways. These compounds can ...
... the synthesis of urea, which is quantitatively the most important route for disposing of nitrogen from the body. • In the second phase of amino acid catabolism, the carbon skeletons of the α-ketoacids are converted to common intermediates of energy producing, metabolic pathways. These compounds can ...
Name ________________________________________ Date __________________ Class Period___________________________________
... 1. What is homeostasis? 2. How would your body respond if you were to leave a warm house and go outside without wearing a warm jacket on a very cold day? 3. How does diffusion help living things maintain homeostasis? 4. What is an example of a process that occurs by diffusion and helps maintain home ...
... 1. What is homeostasis? 2. How would your body respond if you were to leave a warm house and go outside without wearing a warm jacket on a very cold day? 3. How does diffusion help living things maintain homeostasis? 4. What is an example of a process that occurs by diffusion and helps maintain home ...
Motoneuron Muscle Glucose Uptake
... What is the reduction of pyruvate to lactate catalyzed by lactate dehydrogenase? ...
... What is the reduction of pyruvate to lactate catalyzed by lactate dehydrogenase? ...
Chapter 16 - The Citric Acid Cycle
... catabolism of carbohydrates, lipids and amino acids • Intermediates of the cycle are starting points for many biosynthetic reactions • Enzymes of the cycle are in the mitochondria (eukaryotes) or the cytosol of bacteria • Energy of the oxidation reactions is largely conserved as reducing power • Coe ...
... catabolism of carbohydrates, lipids and amino acids • Intermediates of the cycle are starting points for many biosynthetic reactions • Enzymes of the cycle are in the mitochondria (eukaryotes) or the cytosol of bacteria • Energy of the oxidation reactions is largely conserved as reducing power • Coe ...
Chemical Formulas
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
Year 9 Respiration QUICK VERSION
... except Venus (greenhouse effect) • Further from the sun they take longer to orbit (travel slower and have to go further) • Inner planets – small, rocky, few moons • Outer planets – large, mainly gas, lots of moons ...
... except Venus (greenhouse effect) • Further from the sun they take longer to orbit (travel slower and have to go further) • Inner planets – small, rocky, few moons • Outer planets – large, mainly gas, lots of moons ...
Answers to Mastering Concepts Questions
... the lungs; in cellular respiration, it takes place in the cells themselves. 2. How are photosynthesis and cellular respiration interrelated? Each process produces the materials needed for the other process. Photosynthesis produces glucose and oxygen that are required for cellular respiration to func ...
... the lungs; in cellular respiration, it takes place in the cells themselves. 2. How are photosynthesis and cellular respiration interrelated? Each process produces the materials needed for the other process. Photosynthesis produces glucose and oxygen that are required for cellular respiration to func ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.