What is the function of DNA?
... (ribosomal) and tRNA (transfer) • mRNA; carries a copy of the DNA code from the nucleus to the ribosome in the cytoplasm • rRNA; formed at the ribosome alongside protein • tRNA; carries an amino acid to the ribosome in the cytoplasm ...
... (ribosomal) and tRNA (transfer) • mRNA; carries a copy of the DNA code from the nucleus to the ribosome in the cytoplasm • rRNA; formed at the ribosome alongside protein • tRNA; carries an amino acid to the ribosome in the cytoplasm ...
File
... Some water in a solution will break down into H+ and OH These will be in equal number in pure water ...
... Some water in a solution will break down into H+ and OH These will be in equal number in pure water ...
Key to Protein Synthesis Vocabulary
... synthesis in the cytoplasm; a ribosome consists of two ribosomal sununites (the small and the large), each composed of rRNA and protein molecules RNA molecules that function as enzymes. Often ribozymes have associated proteins, although sometimes these proteins only seem to serve a structural role. ...
... synthesis in the cytoplasm; a ribosome consists of two ribosomal sununites (the small and the large), each composed of rRNA and protein molecules RNA molecules that function as enzymes. Often ribozymes have associated proteins, although sometimes these proteins only seem to serve a structural role. ...
Recombinant Human Epiregulin (rh EREG)
... Physical Appearance: Sterile filtered white lyophilized (freeze-dried) powder. Formulation: lyophilized from 0.5mg/ml solution containing 20mM PBS pH-7.4 + 130mM NaCl. The samples of 1µg contain Trehalose 5% (w/vol) for better recovery Solubility: It is recommended to reconstitute the lyophilized rh ...
... Physical Appearance: Sterile filtered white lyophilized (freeze-dried) powder. Formulation: lyophilized from 0.5mg/ml solution containing 20mM PBS pH-7.4 + 130mM NaCl. The samples of 1µg contain Trehalose 5% (w/vol) for better recovery Solubility: It is recommended to reconstitute the lyophilized rh ...
DNA, RNA, and Protein
... Peptide bonds link amino acids together There are 20 essential amino acids found in all living things. Some have modifications. o o o • Amino acids form 1 , 2 & 3 protein structures – Structures are essential to protein function ...
... Peptide bonds link amino acids together There are 20 essential amino acids found in all living things. Some have modifications. o o o • Amino acids form 1 , 2 & 3 protein structures – Structures are essential to protein function ...
Patient Handout
... and hips. Lipotropic, or fat burning, substances include methionine which helps the liver remove fat; inositol, similar to methionine; choline, which distributes cholesterol and prevents it from getting deposited in one part of the body. In some cases, a combination of these may be given. Injections ...
... and hips. Lipotropic, or fat burning, substances include methionine which helps the liver remove fat; inositol, similar to methionine; choline, which distributes cholesterol and prevents it from getting deposited in one part of the body. In some cases, a combination of these may be given. Injections ...
The Aromatic Character of Substituted Tria
... An energy function and parameters (D, F, R0) are assigned to each bond in the molecule. In a similar fashion appropriate functions and parameters are assigned to each type of ...
... An energy function and parameters (D, F, R0) are assigned to each bond in the molecule. In a similar fashion appropriate functions and parameters are assigned to each type of ...
Microbial metabolism
... • There are many important precursor metabolites formed during the central metabolic pathways which are used in biosynthetic (anabolic) pathways. – Fructose 6-phosphate • Generated during glycolysis • Used in the synthesis of peptidoglycan ...
... • There are many important precursor metabolites formed during the central metabolic pathways which are used in biosynthetic (anabolic) pathways. – Fructose 6-phosphate • Generated during glycolysis • Used in the synthesis of peptidoglycan ...
Excitatory Amino Acids Brochure
... Glutamate is the major excitatory neurotransmitter in the brain and dysfunction of glutamate transmission is the likely cause of a variety of diseases including neurodegeneration following cerebral ischemia, Huntington's chorea, amyotrophic lateral sclerosis, epilepsy, spasticity, emesis, chronic pa ...
... Glutamate is the major excitatory neurotransmitter in the brain and dysfunction of glutamate transmission is the likely cause of a variety of diseases including neurodegeneration following cerebral ischemia, Huntington's chorea, amyotrophic lateral sclerosis, epilepsy, spasticity, emesis, chronic pa ...
Transcription and translation
... • EPO boosts production of red blood cells – Lance Armstrong used it. • Concern now that athletes may inject genes to make EPO into their cells • New test can scan for this gene using introns/exons! • A person’s own EPO gene has introns. • An inserted gene would likely lack those introns. So their a ...
... • EPO boosts production of red blood cells – Lance Armstrong used it. • Concern now that athletes may inject genes to make EPO into their cells • New test can scan for this gene using introns/exons! • A person’s own EPO gene has introns. • An inserted gene would likely lack those introns. So their a ...
No Slide Title
... Glu and Gln release NH4+ in the mitochondria of hepatocyte Asp is generated in mitochondrial matrix by transamination and transported into the cytosol of hepatocyte ...
... Glu and Gln release NH4+ in the mitochondria of hepatocyte Asp is generated in mitochondrial matrix by transamination and transported into the cytosol of hepatocyte ...
KEY CONCEPT Enzymes are catalysts for chemical
... Enzymes allow chemical reactions to occur under tightly controlled conditions. • Enzymes are catalysts in living things. ...
... Enzymes allow chemical reactions to occur under tightly controlled conditions. • Enzymes are catalysts in living things. ...
Advanced Placement Chemistry: 1984 Free Response Questions
... (b) Burning coal containing a significant amount of sulfur leads to "acid rain." (c) Perspiring is a mechanism for cooling the body. (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
... (b) Burning coal containing a significant amount of sulfur leads to "acid rain." (c) Perspiring is a mechanism for cooling the body. (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
... A phospholipid replaces the fatty acid at position 1 with a phosphate group that may link to other groups (such as choline) 5. (10 pts) Please do any one of the following three questions: Choice A: Briefly describe the role of the hydrophobic effect on the formation of phospholipid bilayers and mice ...
the ubiquitin system and a putative stimulatory role
... It is not entirely accurate to think of Ub as a simple tag, as Ub does appear to be involved in degradation. The proteasome is the structure that actually does the degrading. Ubiquiton's degradation role may simply be to decrease the rate of dissociation between proteasomes and interacting substrate ...
... It is not entirely accurate to think of Ub as a simple tag, as Ub does appear to be involved in degradation. The proteasome is the structure that actually does the degrading. Ubiquiton's degradation role may simply be to decrease the rate of dissociation between proteasomes and interacting substrate ...
Activity 17 Follow-up
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
L23 HH Glycolysis Citric Acid Cycle e
... Citric Acid Cycle (also known as Kreb cycle/TCA cycle) • If oxygen is present pyruvate is broken down into carbon dioxide and an acetyl group. • This occurs in the central matrix of the mitochondria • The acetyl group that combines with coenzyme A to be transferred to the citric acid cycle as acety ...
... Citric Acid Cycle (also known as Kreb cycle/TCA cycle) • If oxygen is present pyruvate is broken down into carbon dioxide and an acetyl group. • This occurs in the central matrix of the mitochondria • The acetyl group that combines with coenzyme A to be transferred to the citric acid cycle as acety ...
Title - Iowa State University
... b) How many moles of NO are produced by the reaction of 4 moles of copper with excess HNO 3? ...
... b) How many moles of NO are produced by the reaction of 4 moles of copper with excess HNO 3? ...
Gas Exchange
... • Organisms that live in aquatic, marine, or even moist terrestrial environments and which have all tissues within 1 mm of the moist integument, do not have specialized gas exchange structures nor do they require a circulatory system to transport oxygen. In some, such as nemertean worms a circulator ...
... • Organisms that live in aquatic, marine, or even moist terrestrial environments and which have all tissues within 1 mm of the moist integument, do not have specialized gas exchange structures nor do they require a circulatory system to transport oxygen. In some, such as nemertean worms a circulator ...
IUPAC-IUB Commission on Biochemical Nomenclature
... assigned to the most frequently occurring and structurally most simple amino acids. On this basis, the letters A, G, L, P and T are assigned to alanine, glycine, leucine, proline and threonine respectively. 3.3. The assignment of the other abbreviations is more arbitrary. However, certain clues are ...
... assigned to the most frequently occurring and structurally most simple amino acids. On this basis, the letters A, G, L, P and T are assigned to alanine, glycine, leucine, proline and threonine respectively. 3.3. The assignment of the other abbreviations is more arbitrary. However, certain clues are ...
Respiration
... transport chain. • Several chain molecules can use the exergonic flow of electrons to pump H+ from the matrix to the intermembrane space. – This concentration of H+ is the protonmotive force. ...
... transport chain. • Several chain molecules can use the exergonic flow of electrons to pump H+ from the matrix to the intermembrane space. – This concentration of H+ is the protonmotive force. ...
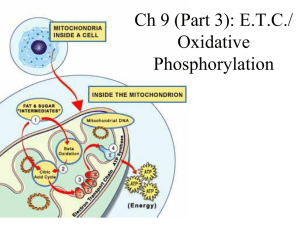
Ch 9: E.T.C./ Oxidative Phosphorylation
... • the groups along the chain alternate between reduced & oxidized states as they accept and donate electrons • each successive group is more electronegative than the group before it, so the electrons are “pulled downhill” towards OXYGEN (the final electron carrier!) ...
... • the groups along the chain alternate between reduced & oxidized states as they accept and donate electrons • each successive group is more electronegative than the group before it, so the electrons are “pulled downhill” towards OXYGEN (the final electron carrier!) ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.