Review Keystone Biology Multiple choice
... What molecules are joined together to make proteins? a. amino acids b. lipids c. nucleic acids d. sugars ...
... What molecules are joined together to make proteins? a. amino acids b. lipids c. nucleic acids d. sugars ...
PS 3 Answers
... An irregularly shaped "shaft" linked to the Fo proton pore rotates relative to the F1 proteins, which are arranged in a ring, when H+ ions flow through Fo. The conformation of each b subunit changes sequentially, as it interacts with the rotating shaft. Each of the 3 b subunits is in a different sta ...
... An irregularly shaped "shaft" linked to the Fo proton pore rotates relative to the F1 proteins, which are arranged in a ring, when H+ ions flow through Fo. The conformation of each b subunit changes sequentially, as it interacts with the rotating shaft. Each of the 3 b subunits is in a different sta ...
biochemistry, cell and molecular biology test
... detaches the cell from focal contacts at the rear. c. (1) Actin polymerization extends the cell forward in the direction of migration; (2) Focal contacts are disassembled at the front of the cell to allow it to move over the substratum (3) Myosin II contraction also detaches the cell from focal cont ...
... detaches the cell from focal contacts at the rear. c. (1) Actin polymerization extends the cell forward in the direction of migration; (2) Focal contacts are disassembled at the front of the cell to allow it to move over the substratum (3) Myosin II contraction also detaches the cell from focal cont ...
The genetic code
... spatially and temporally separated. Transcription occurs in the nucleus to produce a pre-mRNA molecule. The pre-mRNA is typically processed to produce the mature mRNA, which exits the nucleus and is translated in the cytoplasm. ...
... spatially and temporally separated. Transcription occurs in the nucleus to produce a pre-mRNA molecule. The pre-mRNA is typically processed to produce the mature mRNA, which exits the nucleus and is translated in the cytoplasm. ...
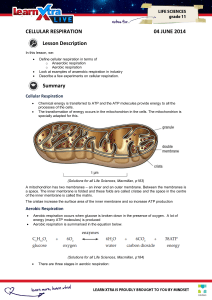
CELLULAR RESPIRATION 04 JUNE 2014 Lesson Description
... Oxidative phosphorylation: takes the energy from the energy-rich hydrogens to make ATP. The energy depleted hydrogens combine with oxygen to make water. This is either breathed out as water vapour or excreted via the kidneys. ...
... Oxidative phosphorylation: takes the energy from the energy-rich hydrogens to make ATP. The energy depleted hydrogens combine with oxygen to make water. This is either breathed out as water vapour or excreted via the kidneys. ...
Name___________________________ Lab #______ Role: Activity
... and amino acids to the mRNA to connect the amino acids together to form a chain. Your protein chain will be complete when you reach the stop codon. There is no amino acid associated with the stop codon. ...
... and amino acids to the mRNA to connect the amino acids together to form a chain. Your protein chain will be complete when you reach the stop codon. There is no amino acid associated with the stop codon. ...
Second Semester Extra Review
... 6. Calculate the pH of a 0.200 M HSO4-1 with a Ka of 1.20 x 10-7. 7. Calculate the Ksp of Fe2O3 which has a solubility of 1.30 g/100g H2O. 8. Calculate the molar solubility of aluminum chloride if the Ksp is 4.7 x 10-8. Chapter 20 & 21 1. What are organic compounds? 2. What is the general formula fo ...
... 6. Calculate the pH of a 0.200 M HSO4-1 with a Ka of 1.20 x 10-7. 7. Calculate the Ksp of Fe2O3 which has a solubility of 1.30 g/100g H2O. 8. Calculate the molar solubility of aluminum chloride if the Ksp is 4.7 x 10-8. Chapter 20 & 21 1. What are organic compounds? 2. What is the general formula fo ...
Proteins
... B. Amino acids are molecules composed of carbon, hydrogen, oxygen, and nitrogen. C. Amino acids all have the same basic structure. They all have a central carbon atom that is bound to the following (Figure 5.1): 1. A single hydrogen atom 2. A carboxylic acid group (-COOH) 3. An amino group (-NH2) 4. ...
... B. Amino acids are molecules composed of carbon, hydrogen, oxygen, and nitrogen. C. Amino acids all have the same basic structure. They all have a central carbon atom that is bound to the following (Figure 5.1): 1. A single hydrogen atom 2. A carboxylic acid group (-COOH) 3. An amino group (-NH2) 4. ...
Amino Acids, Peptides, and Proteins
... The unique sequence of amino acids in a peptide or protein is termed the PRIMARY (1°) STRUCTURE of the Protein. Genes contain the information necessary for the directed synthesis of a protein, for the assembly of the primary structure of the protein. The primary structure contains the information ne ...
... The unique sequence of amino acids in a peptide or protein is termed the PRIMARY (1°) STRUCTURE of the Protein. Genes contain the information necessary for the directed synthesis of a protein, for the assembly of the primary structure of the protein. The primary structure contains the information ne ...
Amylase v1
... • Given organisms from three domains with diverse lifestyles and study sequence differences and their effect on enzyme’s structure and function. • Are the structures of amylase different across organisms? • Relate the identity and percentage similarities in sequences based on clustering in the phylo ...
... • Given organisms from three domains with diverse lifestyles and study sequence differences and their effect on enzyme’s structure and function. • Are the structures of amylase different across organisms? • Relate the identity and percentage similarities in sequences based on clustering in the phylo ...
You Light Up My Life
... • Oxygen combines with spent electrons and H+ to form water - which is used by the cell - it is not released from your ...
... • Oxygen combines with spent electrons and H+ to form water - which is used by the cell - it is not released from your ...
Essay Prompt #1 - Cloudfront.net
... affects the process of diffusion through a membrane _______________________ Max possible = 14 * No points if the lab will not work. **Osmosis: the diffusion of water through a selectively (semi)permeable membrane in the following directions: -from higher water potential toward lower water potential ...
... affects the process of diffusion through a membrane _______________________ Max possible = 14 * No points if the lab will not work. **Osmosis: the diffusion of water through a selectively (semi)permeable membrane in the following directions: -from higher water potential toward lower water potential ...
Enzymes - CEA Workshop Teacher Notes.pptx
... sugars, pepJdes/proteins, DNA, hormones/steroids. • Medicines that are chiral must be made as a single ‘mirror image’ form to avoid undesired side-‐effects. ...
... sugars, pepJdes/proteins, DNA, hormones/steroids. • Medicines that are chiral must be made as a single ‘mirror image’ form to avoid undesired side-‐effects. ...
Chemical Reactions and Reaction Stoichiometry
... The most important commercial process for converting nitrogen from the air into nitrogen-containing compounds is based on the reaction nitrogen gas reacting with hydrogen gas to form ammonia. How many moles of ammonia can be produced from 3 moles of nitrogen and 6 moles of hydrogen? ...
... The most important commercial process for converting nitrogen from the air into nitrogen-containing compounds is based on the reaction nitrogen gas reacting with hydrogen gas to form ammonia. How many moles of ammonia can be produced from 3 moles of nitrogen and 6 moles of hydrogen? ...
CHEM1405 2006-J-4 June 2006 • Draw a Lewis structure and thus
... This hybridisation means that the ‘lone pair’ is in a p-orbital on N and is able to become involved in π bonding with the C=O. This resonance acts to strengthen the N-C bond. ...
... This hybridisation means that the ‘lone pair’ is in a p-orbital on N and is able to become involved in π bonding with the C=O. This resonance acts to strengthen the N-C bond. ...
Part a
... molecules become aligned with the slightly negative ends (–) of other water molecules. Copyright © 2010 Pearson Education, Inc. ...
... molecules become aligned with the slightly negative ends (–) of other water molecules. Copyright © 2010 Pearson Education, Inc. ...
ADM: Facts about Fats
... which contains about 47.5% unsaturated fatty acids, melts at around 42º C, while canola oil, with 93% unsaturated fatty acids, melts at -10º C. Liquid vegetable oils are converted to solid margarines by hydrogenation which adds hydrogen atoms to unsaturated fatty acids and, therefore, increases thei ...
... which contains about 47.5% unsaturated fatty acids, melts at around 42º C, while canola oil, with 93% unsaturated fatty acids, melts at -10º C. Liquid vegetable oils are converted to solid margarines by hydrogenation which adds hydrogen atoms to unsaturated fatty acids and, therefore, increases thei ...
Questions Ch 24
... 3) Ampicillin is a semi-synthetic antibiotic derivative of penicillin G. Ampicillin is acidresistant, whereas penicillin G is not resistant to acid. Why is this important? a) Ampicillin is resistant to β-lactamases. b) Ampicillin can be taken orally. c) Ampicillin is effective against Gram-negative ...
... 3) Ampicillin is a semi-synthetic antibiotic derivative of penicillin G. Ampicillin is acidresistant, whereas penicillin G is not resistant to acid. Why is this important? a) Ampicillin is resistant to β-lactamases. b) Ampicillin can be taken orally. c) Ampicillin is effective against Gram-negative ...
H - IS MU
... high intake of fructose results in increased production of fatty acids and consequently increased production of triacylglycerols ...
... high intake of fructose results in increased production of fatty acids and consequently increased production of triacylglycerols ...
Chapter 6: Proteins
... b) amino acids that the body is unable to synthesize. c) amino acids that the body is able to synthesize in sufficient quantities to meet its needs. d) amino acids such as phenylalanine that the body cannot metabolize. ...
... b) amino acids that the body is unable to synthesize. c) amino acids that the body is able to synthesize in sufficient quantities to meet its needs. d) amino acids such as phenylalanine that the body cannot metabolize. ...
Cells and Energy
... Mitochondria – site of cellular respiration in cells. Glucose – energy source broken down to release ATP. NADH & FADH2 – coenzymes that shuttle electrons from Glycolysis & The Krebs Cycle to the Electron Transport Chain. Glycolysis – begins the breakdown of glucose into two molecules of pyruvate. In ...
... Mitochondria – site of cellular respiration in cells. Glucose – energy source broken down to release ATP. NADH & FADH2 – coenzymes that shuttle electrons from Glycolysis & The Krebs Cycle to the Electron Transport Chain. Glycolysis – begins the breakdown of glucose into two molecules of pyruvate. In ...
The Central Dogma of Molecular Biology
... involves many enzymes: replication 2. the DNA codes for the production of messenger RNA (mRNA) during transcription 3. In eucaryotic cells, the mRNA is processed and migrates from the nucleus to the cytoplasm 4. Messenger RNA carries coded information to the ribosomes. The ribosomes “read” thi ...
... involves many enzymes: replication 2. the DNA codes for the production of messenger RNA (mRNA) during transcription 3. In eucaryotic cells, the mRNA is processed and migrates from the nucleus to the cytoplasm 4. Messenger RNA carries coded information to the ribosomes. The ribosomes “read” thi ...
Bioenergetics, glycolysis, metabolism of monosaccharides and
... Where: ΔGo is the standard free energy change, R is the gas constant (1.987 cal/mol . degree), T is the absolute temperature (K), [A] and [B] are the actual concentrations of the reactant and product, ln represents the natural logarithm. Example: for non-equilibirium conditions (see the figure) gluc ...
... Where: ΔGo is the standard free energy change, R is the gas constant (1.987 cal/mol . degree), T is the absolute temperature (K), [A] and [B] are the actual concentrations of the reactant and product, ln represents the natural logarithm. Example: for non-equilibirium conditions (see the figure) gluc ...
UNIVERSITY OF CALICUT
... glyceraldehyde, examples of epimers, mutarotation and its explanation, anomeric forms, classification of monosaccharides, linear and cyclic structure (glucose, galactose, mannose, ribose and fructose).Reactions and characteristics of aldehyde and keto group, action of acids and alkalies on sugars, r ...
... glyceraldehyde, examples of epimers, mutarotation and its explanation, anomeric forms, classification of monosaccharides, linear and cyclic structure (glucose, galactose, mannose, ribose and fructose).Reactions and characteristics of aldehyde and keto group, action of acids and alkalies on sugars, r ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.