Chemistry Test Review - Greenslime Home Page
... a. the element should have: 14 Protons 14 Neutrons ...
... a. the element should have: 14 Protons 14 Neutrons ...
test 4 study guide - Lindbergh Schools
... I can describe how pressure, heat, and recrystalliztion form metamorphic rock. I can describe how pressurization, heating and recrystalliztion are internal processes of the rock cycle. I can describe where in the geosphere metamorphic rocks are formed. ...
... I can describe how pressure, heat, and recrystalliztion form metamorphic rock. I can describe how pressurization, heating and recrystalliztion are internal processes of the rock cycle. I can describe where in the geosphere metamorphic rocks are formed. ...
File - Mr. L`s Room
... Characterisitcs of Science: Each of these items were covered on the previous study guides: Safety, Scientific Method (Process), Experimental Design, Lab Equipment, and Measurements (including SI Units). S8P1a Atoms and Molecules: See Atoms and the Periodic Table as well. 1. Define atom, element, mol ...
... Characterisitcs of Science: Each of these items were covered on the previous study guides: Safety, Scientific Method (Process), Experimental Design, Lab Equipment, and Measurements (including SI Units). S8P1a Atoms and Molecules: See Atoms and the Periodic Table as well. 1. Define atom, element, mol ...
Unit 3: Properties and States of Matter
... • Main idea – all matter is made up of particles and these particles are always moving to some extent • Crushing this can without using my hands is an example of this ...
... • Main idea – all matter is made up of particles and these particles are always moving to some extent • Crushing this can without using my hands is an example of this ...
Synthesis, Crystal Growth, Structural, Optical, Thermal and
... L-cystine dihydrochloride (C6H12S2N2O4.2HCl) is yet another semiorganic nonlinear optical material. L-Cystine is a sulfur containing amino acid. The chirality of L-cystine enforces noncentrosymmetry of crystal structures of its compounds. This is an important aspect in addition to chemical and confo ...
... L-cystine dihydrochloride (C6H12S2N2O4.2HCl) is yet another semiorganic nonlinear optical material. L-Cystine is a sulfur containing amino acid. The chirality of L-cystine enforces noncentrosymmetry of crystal structures of its compounds. This is an important aspect in addition to chemical and confo ...
Materials Science for Chemical Engineers
... - There are three types of van der Waals interactions: 1) London or Dispersion Forces: instantaneous dipole/induced dipole forces always present between atoms, ions or molecules, but may be overshadowed by strong, primary bonds. ...
... - There are three types of van der Waals interactions: 1) London or Dispersion Forces: instantaneous dipole/induced dipole forces always present between atoms, ions or molecules, but may be overshadowed by strong, primary bonds. ...
Earth`s Waters Section 1–1 Review and Reinforce (p. 17) 1
... 2. Minerals 3. crystallize 4. veins 5. In general, minerals can form in two ways: through crystallization of melted materials, and through crystallization of materials dissolved in water. 6. Large crystals are likely to form when magma cools slowly, such as deep underground. Small crystals are likel ...
... 2. Minerals 3. crystallize 4. veins 5. In general, minerals can form in two ways: through crystallization of melted materials, and through crystallization of materials dissolved in water. 6. Large crystals are likely to form when magma cools slowly, such as deep underground. Small crystals are likel ...
Introduction to Mineralogy Dr. Tark Hamilton Chapter 3
... The Phase Rule & Heterogeneous Equilibria ...
... The Phase Rule & Heterogeneous Equilibria ...
Exam 2
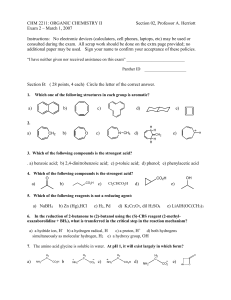
... Org II exam, p.2 Section A (48 points, 4 each – answer 12 of the 13 questions, marking clearly the one you choose to omit; if none is marked, the last answer will be omitted) Give the major organic product(s) of the following reactions, including stereochemistry if appropriate. ...
... Org II exam, p.2 Section A (48 points, 4 each – answer 12 of the 13 questions, marking clearly the one you choose to omit; if none is marked, the last answer will be omitted) Give the major organic product(s) of the following reactions, including stereochemistry if appropriate. ...
PHYSICAL PROPERTIES - can observe w/o changing the
... oxidation, burning, combustibility, flammability, toxicity, electronegativity, reactivity with other substances, pH, corrosiveness ...
... oxidation, burning, combustibility, flammability, toxicity, electronegativity, reactivity with other substances, pH, corrosiveness ...
Introduction to Mineralogy
... Minerals are chemical compounds that form from natural earth processes Minerals are basic building blocks of rocks Rocks provide record of earth history and are formed by earth processes Geologists ...
... Minerals are chemical compounds that form from natural earth processes Minerals are basic building blocks of rocks Rocks provide record of earth history and are formed by earth processes Geologists ...
Final Exam review semester 1
... hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
... hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
Separation and Purification Methods
... pressure, or vacuum distillation, brings many advantages. For example, consider a liquid that has a boiling point of 180◦ C at atmospheric pressure (760 Torr). If you were to attempt that distillation, you would not be surprised to observe charring of the compound at such elevated temperature. Fortu ...
... pressure, or vacuum distillation, brings many advantages. For example, consider a liquid that has a boiling point of 180◦ C at atmospheric pressure (760 Torr). If you were to attempt that distillation, you would not be surprised to observe charring of the compound at such elevated temperature. Fortu ...
Elements compounds and mixtures
... • If homogeneous matter can be separated by physical means, then the matter is a mixture. • If homogeneous matter cannot be separated by physical means, then the matter is a pure substance. • If a pure substance can be decomposed into something else, then the substance is a compound. • If a pure sub ...
... • If homogeneous matter can be separated by physical means, then the matter is a mixture. • If homogeneous matter cannot be separated by physical means, then the matter is a pure substance. • If a pure substance can be decomposed into something else, then the substance is a compound. • If a pure sub ...
SC71 Chemistry
... Formulate predictions, questions, or hypotheses based on observations. Evaluate appropriate resources. PO 1. Evaluate scientific information for relevance to a given problem. (See R09-S3C1, R10-S3C1, R11S3C1, and R12-S3C1) PO 2. Develop questions from observations that transition into testable hypot ...
... Formulate predictions, questions, or hypotheses based on observations. Evaluate appropriate resources. PO 1. Evaluate scientific information for relevance to a given problem. (See R09-S3C1, R10-S3C1, R11S3C1, and R12-S3C1) PO 2. Develop questions from observations that transition into testable hypot ...
Basic Concepts of the Gas Phase
... Alfons Buekens was born in Aalst, Belgium; he obtained his M.Sc. (1964) and his Ph.D (1967) at Ghent University (RUG) and received the K.V.I.V.-Award (1965), the Robert De Keyser Award (Belgian Shell Co., 1968), the Körber Foundation Award (1988) and the Coca Cola Foundation Award (1989). Dr. Bueken ...
... Alfons Buekens was born in Aalst, Belgium; he obtained his M.Sc. (1964) and his Ph.D (1967) at Ghent University (RUG) and received the K.V.I.V.-Award (1965), the Robert De Keyser Award (Belgian Shell Co., 1968), the Körber Foundation Award (1988) and the Coca Cola Foundation Award (1989). Dr. Bueken ...
Stuff Matters Handout
... Elements and compounds can move from one physical state to another and not change their basic atomic parts. Oxygen (O2) as a gas still has the same properties as liquid oxygen. The liquid state is colder and denser, but the molecules (the basic parts) are still the same. Water (H2O) is another examp ...
... Elements and compounds can move from one physical state to another and not change their basic atomic parts. Oxygen (O2) as a gas still has the same properties as liquid oxygen. The liquid state is colder and denser, but the molecules (the basic parts) are still the same. Water (H2O) is another examp ...
Pure substances
... • Distillation is an effective method of separating a mixture of liquids or to purify a liquid with solid particles dissolved in it − Uses the differences in the boiling points of ...
... • Distillation is an effective method of separating a mixture of liquids or to purify a liquid with solid particles dissolved in it − Uses the differences in the boiling points of ...
What is matter?
... Volume is a measurement of how much space an object takes up. Volume of a rectangular prism can be found by multiplying length x width x height using cm3. Volume of a liquid is measured in a graduated cylinder using the metric unit milliliters. Volume of irregular objects can be found by dropping th ...
... Volume is a measurement of how much space an object takes up. Volume of a rectangular prism can be found by multiplying length x width x height using cm3. Volume of a liquid is measured in a graduated cylinder using the metric unit milliliters. Volume of irregular objects can be found by dropping th ...
Structural basis for the fast phase change of DVD-RAM
... structure by Chen et al. and Yamada et al., respectively. They reported that these materials show a high phase-stability of the amorphous phase and a very short crystallization time. These approaches discovered the way to develop new phase-change rewritable materials and led to the discovery in 1987 ...
... structure by Chen et al. and Yamada et al., respectively. They reported that these materials show a high phase-stability of the amorphous phase and a very short crystallization time. These approaches discovered the way to develop new phase-change rewritable materials and led to the discovery in 1987 ...
Stoichiometry - hrsbstaff.ednet.ns.ca
... double bonds), alkynes (one triple bond), nonsubstituted cycloalkanes and cycloalkenes defining and being able to give examples of saturated and unsaturated hydrocarbons being able to name all the prefixes for one to ten carbons in a compound or alkyl group writing names, molecular formulas, a ...
... double bonds), alkynes (one triple bond), nonsubstituted cycloalkanes and cycloalkenes defining and being able to give examples of saturated and unsaturated hydrocarbons being able to name all the prefixes for one to ten carbons in a compound or alkyl group writing names, molecular formulas, a ...
(1) Dissolves, accompanied by evolution of flammable gas (2
... SELECT TWO OF THE FOUR ESSAY QUESTIONS, NUMBERED 6 THROUGH 9. (Additional essays will not be scored.) ...
... SELECT TWO OF THE FOUR ESSAY QUESTIONS, NUMBERED 6 THROUGH 9. (Additional essays will not be scored.) ...