Chemistry Unit Test Review
... • C. They are in the same period. • D. They are not chemically similar. ...
... • C. They are in the same period. • D. They are not chemically similar. ...
Review of the Dissertation of Elena S. Zhitova «Crystal Chemistry of
... belongs to the layered double hydroxides (LDHs), a large class of natural and synthetic compounds. To date, 44 minerals have been described as natural examples of LDH phases; they are commonly known as the ‘hydrotalcites’ or ‘hydrotalcite group’ of minerals. Scientifically, these minerals are intere ...
... belongs to the layered double hydroxides (LDHs), a large class of natural and synthetic compounds. To date, 44 minerals have been described as natural examples of LDH phases; they are commonly known as the ‘hydrotalcites’ or ‘hydrotalcite group’ of minerals. Scientifically, these minerals are intere ...
Chemistry Review: Unit2 - Menno Simons Christian School
... 3) Use particle theory to explain what a solution is. Contains at least two different types of particles: the solvent particles(more numerous) and the solute particles (less numerous) which are evenly mixed. __________________________________________________________________________________________ _ ...
... 3) Use particle theory to explain what a solution is. Contains at least two different types of particles: the solvent particles(more numerous) and the solute particles (less numerous) which are evenly mixed. __________________________________________________________________________________________ _ ...
PHASES OF MATTER -4 PHASE DIAGRAMS
... • Points A-B and C-D indicate even as heat is applied to the system the phase does not change – the energy is used to break the IM forces (dynamic equilibrium). ...
... • Points A-B and C-D indicate even as heat is applied to the system the phase does not change – the energy is used to break the IM forces (dynamic equilibrium). ...
chem100c1f
... What is Matter • Matter: Anything that has a mass and volume. The opposite of matter is energy. • Matter and Energy is ...
... What is Matter • Matter: Anything that has a mass and volume. The opposite of matter is energy. • Matter and Energy is ...
Chemistry: Matter and Change
... • Define physical change and list several common physical changes. • Define chemical change and list several indications that a chemical change has taken place. • Apply the law of conservation of mass to chemical ...
... • Define physical change and list several common physical changes. • Define chemical change and list several indications that a chemical change has taken place. • Apply the law of conservation of mass to chemical ...
Material Selection - Web Services Overview
... • Most metals can be welded but with a wide range of methods and very different costs. Low alloy steels cost the least, high strength steels cost more, and tool steels are most expensive to weld. • Some materials require pre and post-weld heat treatment. • Welding induces distortion in parts, furthe ...
... • Most metals can be welded but with a wide range of methods and very different costs. Low alloy steels cost the least, high strength steels cost more, and tool steels are most expensive to weld. • Some materials require pre and post-weld heat treatment. • Welding induces distortion in parts, furthe ...
Physical and Chemical Properties
... • There are over one hundred different types of atoms and they oftentimes combine to make new substances known as molecules and compounds Molecule ...
... • There are over one hundred different types of atoms and they oftentimes combine to make new substances known as molecules and compounds Molecule ...
ionic bond. - cloudfront.net
... • Ionic Compound- composed of + and ions combined so charges are equal. (Metal + nonmetal) • Formula Unit- simplest group of atoms form an ionic compound. ...
... • Ionic Compound- composed of + and ions combined so charges are equal. (Metal + nonmetal) • Formula Unit- simplest group of atoms form an ionic compound. ...
1.2 Properties and Changes of Matter
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
1.2 PowerPoint
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
CSI Case Profile The victim in the following case is a 35
... late that he was “popping painkillers like candy”. Certain painkillers are even more dangerous in large quantities than others. One indication that this may be the cause of death was a partially full acetaminophen bottle found on the scene. Chemical analysis of one pill (1454.10 g) revealed 924.08 g ...
... late that he was “popping painkillers like candy”. Certain painkillers are even more dangerous in large quantities than others. One indication that this may be the cause of death was a partially full acetaminophen bottle found on the scene. Chemical analysis of one pill (1454.10 g) revealed 924.08 g ...
Solutions - Seattle Central
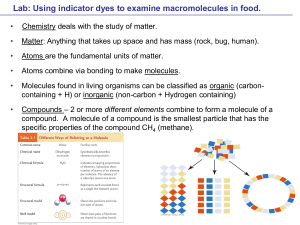
... Matter: Anything that takes up space and has mass (rock, bug, human). ...
... Matter: Anything that takes up space and has mass (rock, bug, human). ...
Bonding Nomenclature Notes
... 1. Polar Covalent Bond -one atom in a molecule is significantly more electronegative -This causes a slight positive and negative charge on a molecule. 2. Nonpolar Covalent Bond -electrons are shared ...
... 1. Polar Covalent Bond -one atom in a molecule is significantly more electronegative -This causes a slight positive and negative charge on a molecule. 2. Nonpolar Covalent Bond -electrons are shared ...
Bonding in Atoms
... the molecule as well as the amount of atoms in the molecule • A formula unit is expressed in the smallest whole number ratio for the molecule • Examples: ...
... the molecule as well as the amount of atoms in the molecule • A formula unit is expressed in the smallest whole number ratio for the molecule • Examples: ...
HIBBING COMMUNITY COLLEGE
... 34. describe what the pH scale measures and what a buffer does. 35. calculate the pH of solutions. 36. identify the different intermolecular forces and how they affect the physical properties of a substance. 37. explain how water dissolves certain compounds. 38. explain on a molecular level why sol ...
... 34. describe what the pH scale measures and what a buffer does. 35. calculate the pH of solutions. 36. identify the different intermolecular forces and how they affect the physical properties of a substance. 37. explain how water dissolves certain compounds. 38. explain on a molecular level why sol ...
Biochemistry Introduction day 1
... Isotopes: Atoms of an element that have the same number of protons but a different number of neutrons. Ex: Oxygen usually has 8 neutrons but 9 and 10 neutrons can be found in some oxygen atoms. Some isotopes are unstable in the nucleus which makes it more likely to decay and release energy. This i ...
... Isotopes: Atoms of an element that have the same number of protons but a different number of neutrons. Ex: Oxygen usually has 8 neutrons but 9 and 10 neutrons can be found in some oxygen atoms. Some isotopes are unstable in the nucleus which makes it more likely to decay and release energy. This i ...
Students will review concepts from their quiz and then correct it at
... A pure substance containing two or more kinds of __atoms__. The atoms are ___chemically___ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds ___cannot___ be separated by physical means ...
... A pure substance containing two or more kinds of __atoms__. The atoms are ___chemically___ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds ___cannot___ be separated by physical means ...
Metric Unit – Chapter 1
... Oxygen and hydrogen are 2 of the more than 100 known elements. Compound = a substance that contains _______________________________ _____________________________________________ in a fixed proportion. Physical methods that are used to separate mixtures cannot be used to break a compound into sim ...
... Oxygen and hydrogen are 2 of the more than 100 known elements. Compound = a substance that contains _______________________________ _____________________________________________ in a fixed proportion. Physical methods that are used to separate mixtures cannot be used to break a compound into sim ...
Matter, Mass and Weight
... Energy of an isolated system always remains constant. Energy may be converted from one form to another, but cannot be created or destroyed. A system can exchange its energy with its surrounding in two forms: heat and work. When a gas is in contact with an object at higher temperature, energy flows i ...
... Energy of an isolated system always remains constant. Energy may be converted from one form to another, but cannot be created or destroyed. A system can exchange its energy with its surrounding in two forms: heat and work. When a gas is in contact with an object at higher temperature, energy flows i ...
form revision a
... There are two types of compound. Covalent compounds form when non-metal atoms form covalent bonds by sharing their outer electrons. Covalent compounds exist as molecules. Ionic compounds form when metal atoms join to non-metal atoms by transferring electron(s) from the metal to the non-metal. The re ...
... There are two types of compound. Covalent compounds form when non-metal atoms form covalent bonds by sharing their outer electrons. Covalent compounds exist as molecules. Ionic compounds form when metal atoms join to non-metal atoms by transferring electron(s) from the metal to the non-metal. The re ...
Unit 2 Review: Chemistry - Mr. Hoover's Science Classes
... Non-metals generally have these properties: Non-conductor of electricity in its solid form At room temperature most are gasses (11) or solids (5) and only one is liquid. Solids are brittle and lack the lustre of metals ...
... Non-metals generally have these properties: Non-conductor of electricity in its solid form At room temperature most are gasses (11) or solids (5) and only one is liquid. Solids are brittle and lack the lustre of metals ...
chapter 8 ceramic/metal nanocomposites
... Freeze drying or lyophilization is a drying method where the solvent is frozen prior to drying and is then sublimed, i.e., passed to the gas phase directly from the solid phase, below the melting point of the solvent. It is increasingly applied to dry foods, beyond its already classical pharmaceutic ...
... Freeze drying or lyophilization is a drying method where the solvent is frozen prior to drying and is then sublimed, i.e., passed to the gas phase directly from the solid phase, below the melting point of the solvent. It is increasingly applied to dry foods, beyond its already classical pharmaceutic ...