Solving Protein Structures
... one wavelength is used to collect diffraction data, whereas MAD uses up to three different wavelengths. In addition to experimental methods used for obtaining initial phase information, there are computational methods such as molecular replacement (MR) and direct Ab initio (direct) calculations. The ...
... one wavelength is used to collect diffraction data, whereas MAD uses up to three different wavelengths. In addition to experimental methods used for obtaining initial phase information, there are computational methods such as molecular replacement (MR) and direct Ab initio (direct) calculations. The ...
Crystallography: An introduction
... Rwork/Rfree: difference < 0.05, Rwork≈ resolution/10 Deviations of known geometry waters: at 2 Å ≈ 1 water/residue, at > 3Å usually none ...
... Rwork/Rfree: difference < 0.05, Rwork≈ resolution/10 Deviations of known geometry waters: at 2 Å ≈ 1 water/residue, at > 3Å usually none ...
MIN faculty Department: Physics Seminar/Institute
... crystals and other systems - Work with the AXSIS team on time-resolved studies that combine X-ray diffraction and spectroscopy measurements Requirements: - A doctoral degree in Physics, Chemistry, Engineering, or related field ...
... crystals and other systems - Work with the AXSIS team on time-resolved studies that combine X-ray diffraction and spectroscopy measurements Requirements: - A doctoral degree in Physics, Chemistry, Engineering, or related field ...
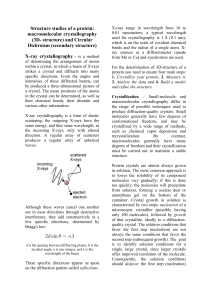
Structure studies of a protein: macromolecular crystallography (3D
... same energy, and thus same wavelength, as the incoming X-rays, only with altered direction. A regular array of scatterers produces a regular array of spherical waves. Although these waves cancel one another out in most directions through destructive interference, they add constructively in a few spe ...
... same energy, and thus same wavelength, as the incoming X-rays, only with altered direction. A regular array of scatterers produces a regular array of spherical waves. Although these waves cancel one another out in most directions through destructive interference, they add constructively in a few spe ...
Cancer Research Postdoctoral Fellow The Protein
... physical chemistry and/or physics with excellent analytical and computational skills. The ideal candidates should have a number of years of research experience in the relevant fields with strong problem-solving skills, be willing to work cooperatively with teams of structural biologists and biologis ...
... physical chemistry and/or physics with excellent analytical and computational skills. The ideal candidates should have a number of years of research experience in the relevant fields with strong problem-solving skills, be willing to work cooperatively with teams of structural biologists and biologis ...
Structure studies of a protein: macromolecular crystallography (3D
... X-ray crystallography - is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and diffracts into many specific directions. From the angles and intensities of these diffracted beams, can be produced a three-dimensional picture of a crystal. ...
... X-ray crystallography - is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and diffracts into many specific directions. From the angles and intensities of these diffracted beams, can be produced a three-dimensional picture of a crystal. ...
Globular proteins
... When a beam of X-ray of a given wave length falls on a crystal, the xrays are diffracted by the electrons of various atoms of the crystal. The diffracted X-rays are recorded on a photographic film or x-ray film by producing a pattern of spots with various intensities. By analysis of the x-ray diffra ...
... When a beam of X-ray of a given wave length falls on a crystal, the xrays are diffracted by the electrons of various atoms of the crystal. The diffracted X-rays are recorded on a photographic film or x-ray film by producing a pattern of spots with various intensities. By analysis of the x-ray diffra ...
X-ray Free-Electron Lasers – a bright future for structural biology
... Protein crystallography using synchrotron radiation sources has had tremendous impact on biology, having yielded the structures of thousands of proteins and given detailed insight into their working mechanisms. However, the technique is limited by the requirement for macroscopic crystals, which can ...
... Protein crystallography using synchrotron radiation sources has had tremendous impact on biology, having yielded the structures of thousands of proteins and given detailed insight into their working mechanisms. However, the technique is limited by the requirement for macroscopic crystals, which can ...
Information about the Panalytical X*Pert Pro Multipurpose
... High Score Plus in conjunction with the ICDD Powder Diffraction File 2 Database (1999), ICDD Powder Diffraction File 4 - Minerals (2012), the American Mineralogist Crystal Structure Database (March 2010) and the Crystallography Open Database (February 2012; www.crystallography.net) ...
... High Score Plus in conjunction with the ICDD Powder Diffraction File 2 Database (1999), ICDD Powder Diffraction File 4 - Minerals (2012), the American Mineralogist Crystal Structure Database (March 2010) and the Crystallography Open Database (February 2012; www.crystallography.net) ...
BBA IInd SEMESTER EXAMINATION 2008-09
... Enumerate the characteristics of various crystal systems. What is meant by crystal imperfections? Distinguish between ionic and Vander Waals crystals. Explain Bragg’s law for X-ray diffraction in crystals. Explain the significance of Brillouin zones with particular reference to any cubic lattice. Wh ...
... Enumerate the characteristics of various crystal systems. What is meant by crystal imperfections? Distinguish between ionic and Vander Waals crystals. Explain Bragg’s law for X-ray diffraction in crystals. Explain the significance of Brillouin zones with particular reference to any cubic lattice. Wh ...
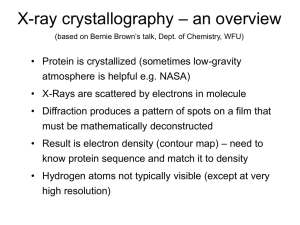
1. Overview
... • Slowly eliminate water from the protein • Add molecules that compete with the protein for water (3 types: salts, organic solvents, PEGs) • Trial and error • Most crystals ~50% solvent • Crystals may be very fragile ...
... • Slowly eliminate water from the protein • Add molecules that compete with the protein for water (3 types: salts, organic solvents, PEGs) • Trial and error • Most crystals ~50% solvent • Crystals may be very fragile ...
X‐ray diffraction: Determining lattice constants of crystal structure
... Thus we have that d = ao/2, ...
... Thus we have that d = ao/2, ...
X-ray Diffraction
... When x-rays were discovered in 1895 by Wilhelm Conrad Röntgen (the first Nobel laureate in Physics1901), their exact nature was not known. The question posed at the time was "are x-rays particles or are they waves like visible light?" Showing that xrays could diffract would prove that x-rays have a ...
... When x-rays were discovered in 1895 by Wilhelm Conrad Röntgen (the first Nobel laureate in Physics1901), their exact nature was not known. The question posed at the time was "are x-rays particles or are they waves like visible light?" Showing that xrays could diffract would prove that x-rays have a ...
X RAY CRYSTALLOGRAPHY
... If all the waves in the bundle are in phase, that is their crests and troughs occur at exactly the same position (the same as being an integer number of wavelengths out of phase, nλ, n = 1, 2, 3, 4, etc.), the waves will interfere with one another and their amplitudes will add together to produce a ...
... If all the waves in the bundle are in phase, that is their crests and troughs occur at exactly the same position (the same as being an integer number of wavelengths out of phase, nλ, n = 1, 2, 3, 4, etc.), the waves will interfere with one another and their amplitudes will add together to produce a ...
The Macromolecular X-ray Crystallography (MX) ESRF Tutorial
... experiment is a 3-dimensional map showing the distribution of electrons in the structure. ...
... experiment is a 3-dimensional map showing the distribution of electrons in the structure. ...
XrayDiffraction
... Therefore if we know Fhkl and (for each h, k, l) we can compute for all values of X, Y, and Z and plot the values obtained to give a threedimensional electron density map. Then, assuming atoms to be at the centres of the electron density peaks, we would have the entire structure. ...
... Therefore if we know Fhkl and (for each h, k, l) we can compute for all values of X, Y, and Z and plot the values obtained to give a threedimensional electron density map. Then, assuming atoms to be at the centres of the electron density peaks, we would have the entire structure. ...
Powder X-Ray Diffraction
... identification. Once the material has been identified, X-ray crystallography may be used to determine its structure, i.e. how the atoms pack together in the crystalline state and what the interatomic distance and angle are etc. Xray diffraction is one of the most important characterization tools use ...
... identification. Once the material has been identified, X-ray crystallography may be used to determine its structure, i.e. how the atoms pack together in the crystalline state and what the interatomic distance and angle are etc. Xray diffraction is one of the most important characterization tools use ...
Why do scientists grow crystals? - Bryn Mawr School Faculty Web
... Substances grown in crystals are extremely pure. These are insulin crystals, grown to purify the insulin used by diabetics ...
... Substances grown in crystals are extremely pure. These are insulin crystals, grown to purify the insulin used by diabetics ...
Lesson 21 - MsReenChemistry
... determining the arrangement of atoms within a crystal structure. Substances including inorganic salts and minerals, semiconductors, and organic and biological compounds can form crystals under suitable and specific conditions. This method of structure determination has provided the most reliable evi ...
... determining the arrangement of atoms within a crystal structure. Substances including inorganic salts and minerals, semiconductors, and organic and biological compounds can form crystals under suitable and specific conditions. This method of structure determination has provided the most reliable evi ...
X-ray crystallography

X-ray crystallography is a tool used for identifying the atomic and molecular structure of a crystal, in which the crystalline atoms cause a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder and various other information.Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various materials, especially minerals and alloys. The method also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystallography is still the chief method for characterizing the atomic structure of new materials and in discerning materials that appear similar by other experiments. X-ray crystal structures can also account for unusual electronic or elastic properties of a material, shed light on chemical interactions and processes, or serve as the basis for designing pharmaceuticals against diseases.In a single-crystal X-ray diffraction measurement, a crystal is mounted on a goniometer. The goniometer is used to position the crystal at selected orientations. The crystal is bombarded with a finely focused monochromatic beam of X-rays, producing a diffraction pattern of regularly spaced spots known as reflections. The two-dimensional images taken at different rotations are converted into a three-dimensional model of the density of electrons within the crystal using the mathematical method of Fourier transforms, combined with chemical data known for the sample. Poor resolution (fuzziness) or even errors may result if the crystals are too small, or not uniform enough in their internal makeup.X-ray crystallography is related to several other methods for determining atomic structures. Similar diffraction patterns can be produced by scattering electrons or neutrons, which are likewise interpreted by Fourier transformation. If single crystals of sufficient size cannot be obtained, various other X-ray methods can be applied to obtain less detailed information; such methods include fiber diffraction, powder diffraction and (if the sample is not crystallized) small-angle X-ray scattering (SAXS).If the material under investigation is only available in the form of nanocrystalline powders or suffers from poor crystallinity, the methods of electron crystallography can be applied for determining the atomic structure.For all above mentioned X-ray diffraction methods, the scattering is elastic; the scattered X-rays have the same wavelength as the incoming X-ray. By contrast, inelastic X-ray scattering methods are useful in studying excitations of the sample, rather than the distribution of its atoms.