Benzene, amines, amino acids and polymers File
... STRUCTURE OF BENZENE Primary analysis revealed benzene had... an ...
... STRUCTURE OF BENZENE Primary analysis revealed benzene had... an ...
The Synthesis of Ferrocene
... name which arose because they participated in reactions similar to those of aromatic molecules. For obvious reasons complexes in which a metal atom was found between two parallel carbocyclic rings became known as "sandwich" compounds. James E. Huheey, Ellen A. Keiter and Richard L. Keiter Inorganic ...
... name which arose because they participated in reactions similar to those of aromatic molecules. For obvious reasons complexes in which a metal atom was found between two parallel carbocyclic rings became known as "sandwich" compounds. James E. Huheey, Ellen A. Keiter and Richard L. Keiter Inorganic ...
DCC-promoted peptide coupling
... amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and di ...
... amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and di ...
nanyang technological university entrance examination syllabus for
... Effect of concentration, temperature, and catalysts on reaction rate Homogeneous and heterogeneous catalysis Enzymes as biological catalysts ...
... Effect of concentration, temperature, and catalysts on reaction rate Homogeneous and heterogeneous catalysis Enzymes as biological catalysts ...
- Opus: Online Publications Store
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
Lecture 3-edited
... 1.3.2 Chromic Acid Oxidation (Jones oxidation) The combination of CrO3 and sulfuric acid is often referred as Jones reagent, and the oxidation of alcohols with this reagent in acetone is called Jones oxidation. The reagent is selective as it is useful for the oxidation of alcohols, which contain car ...
... 1.3.2 Chromic Acid Oxidation (Jones oxidation) The combination of CrO3 and sulfuric acid is often referred as Jones reagent, and the oxidation of alcohols with this reagent in acetone is called Jones oxidation. The reagent is selective as it is useful for the oxidation of alcohols, which contain car ...
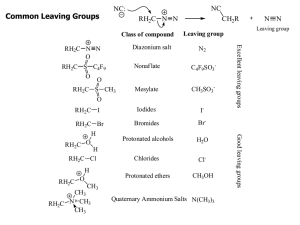
Common Leaving Groups
... E1 vs. E2 vs. SN1 vs. SN2 - Summary •As a general rule, elimination reactions can always compete with substitution reactions. We can, however, alter the reaction conditions to favour one process over another. •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H ...
... E1 vs. E2 vs. SN1 vs. SN2 - Summary •As a general rule, elimination reactions can always compete with substitution reactions. We can, however, alter the reaction conditions to favour one process over another. •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H ...
Oxidation of Benzyl Ethers to Benzoate Esters Using a Novel
... other aqueous solvent mixtures (Thottumkara and Vinod, 2002). Several pertinent observations were made from our initial oxidation studies. It was observed that mIBX tolerates a variety of functional groups during oxidation, and over-oxidation of alcohols is never observed, even when electron rich su ...
... other aqueous solvent mixtures (Thottumkara and Vinod, 2002). Several pertinent observations were made from our initial oxidation studies. It was observed that mIBX tolerates a variety of functional groups during oxidation, and over-oxidation of alcohols is never observed, even when electron rich su ...
14_11_15.html
... The polyethylene formed under Ziegler's conditions is called high-density polyethylene and has, in many ways, more desirable properties than the polyethylene formed by free-radical polymerization. ...
... The polyethylene formed under Ziegler's conditions is called high-density polyethylene and has, in many ways, more desirable properties than the polyethylene formed by free-radical polymerization. ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.