
Preface (PDF, 24 Pages, 5.7 MB)
... permission, in this textbook appear on the appropriate page within the text or on p. P-1. Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publication is protected by Copyright, and p ermission should be obtained from the ...
... permission, in this textbook appear on the appropriate page within the text or on p. P-1. Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publication is protected by Copyright, and p ermission should be obtained from the ...
Chapter 17
... system, they are available to attack a strong electrophile to give a carbocation. This resonance-stabilized carbocation is called a sigma complex because the electrophile is joined to the benzene ring by a new sigma bond. Aromaticity is regained by loss of a proton. Chapter 17 ...
... system, they are available to attack a strong electrophile to give a carbocation. This resonance-stabilized carbocation is called a sigma complex because the electrophile is joined to the benzene ring by a new sigma bond. Aromaticity is regained by loss of a proton. Chapter 17 ...
Downloadable Full Text - DSpace@MIT
... provide an efficient route to highly functionalized synthons from a diverse range of inexpensive, readily available commercial starting materials, such as aldehydes, ketones, imines, epoxides with alkynes, allenes, and alkenes.1 The exact mechanism of many of these processes is still unknown and var ...
... provide an efficient route to highly functionalized synthons from a diverse range of inexpensive, readily available commercial starting materials, such as aldehydes, ketones, imines, epoxides with alkynes, allenes, and alkenes.1 The exact mechanism of many of these processes is still unknown and var ...
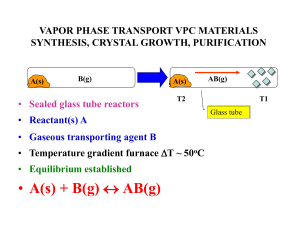
vapor phase transport vpc materials synthesis, crystal growth
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
Organic Chemistry I-2 Ans Chapter 7 Free Radical Answers 1
... 6. Give the product of the reaction of excess benzene with each of the following reagents: a. isobutyl chloride + AlCl3 b. neopentyl chloride + AlCl3 c. propene + HF d. dichloromethane + AlCl3 ...
... 6. Give the product of the reaction of excess benzene with each of the following reagents: a. isobutyl chloride + AlCl3 b. neopentyl chloride + AlCl3 c. propene + HF d. dichloromethane + AlCl3 ...
A-level Chemistry Question paper Unit 04 - Kinetics, Equilibria
... an inert solvent in the presence of a small amount of concentrated sulfuric acid. The equilibrium mixture formed contained 1.80 mol of DEM in a total volume, V dm3, of solution. Calculate the amount (in moles) of A, of ethanol and of water in this equilibrium ...
... an inert solvent in the presence of a small amount of concentrated sulfuric acid. The equilibrium mixture formed contained 1.80 mol of DEM in a total volume, V dm3, of solution. Calculate the amount (in moles) of A, of ethanol and of water in this equilibrium ...
Asymmetric Glycine Enolate Aldol Reactions
... H, NH), 3.85-4.60 (m,3 H, NCHCH,), 2.88 (m,2 H, PhCH,); I3C reaction mixture was stirred at 0 OC for an additional 15 min and then N M R (22.5 MHz, CDCI,) d 159.9, 136.0, 129.1, 128.8, 127.0, 69.4, 53.6, quenched by the addition of 0.56 mL of water, 0.56 mL of aqueous 15% 41; [aID+4.9' (EtOH, c 1.10 ...
... H, NH), 3.85-4.60 (m,3 H, NCHCH,), 2.88 (m,2 H, PhCH,); I3C reaction mixture was stirred at 0 OC for an additional 15 min and then N M R (22.5 MHz, CDCI,) d 159.9, 136.0, 129.1, 128.8, 127.0, 69.4, 53.6, quenched by the addition of 0.56 mL of water, 0.56 mL of aqueous 15% 41; [aID+4.9' (EtOH, c 1.10 ...
HYPERVALENT IODINE IN CARBON-CARBON BOND
... iodonium ylides and generation of benzynes and their cycloaddition reactions.15 A common theme in many of these methods is the generation of high energy transient species such as the phenoxenium cation and free carbenes. Oxidative cyclization is by far the most developed of these approaches and has ...
... iodonium ylides and generation of benzynes and their cycloaddition reactions.15 A common theme in many of these methods is the generation of high energy transient species such as the phenoxenium cation and free carbenes. Oxidative cyclization is by far the most developed of these approaches and has ...
Support material for lesson planning
... At the end of this document you will find a table containing the full set of specification statements, which can be copied and pasted into e.g. your scheme of work. Note that updates to the specification may be made from time to time. Check the Chemistry A qualification page for the definitive, up t ...
... At the end of this document you will find a table containing the full set of specification statements, which can be copied and pasted into e.g. your scheme of work. Note that updates to the specification may be made from time to time. Check the Chemistry A qualification page for the definitive, up t ...
T_AllylCF3paperBM[5]
... ArH led to CF3-alkenes Ar(Ar)CHCH=CHCF3 in 48-75% yields. The mechanisms of the transformations are discussed. ...
... ArH led to CF3-alkenes Ar(Ar)CHCH=CHCF3 in 48-75% yields. The mechanisms of the transformations are discussed. ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.






















![T_AllylCF3paperBM[5]](http://s1.studyres.com/store/data/003584459_1-3decab572f7fca68901a941affab18ea-300x300.png)