Slide 1
... other element towards it. This apparent charge is called an oxidation number, which is the charge it would have if it gained or lost electrons. ...
... other element towards it. This apparent charge is called an oxidation number, which is the charge it would have if it gained or lost electrons. ...
Mechanism of intra - Chemical Engineering Labs
... On Brønsted acid catalysts (H-MFI) [4,5,19], the initial coupling of alkanals leads to larger oxygenates, which undergo secondary reactions of aromatization, alkylation/dealkylation, and cracking. At higher temperatures (e.g., 673 K), these secondary reactions occur much faster than the initial alka ...
... On Brønsted acid catalysts (H-MFI) [4,5,19], the initial coupling of alkanals leads to larger oxygenates, which undergo secondary reactions of aromatization, alkylation/dealkylation, and cracking. At higher temperatures (e.g., 673 K), these secondary reactions occur much faster than the initial alka ...
aq - Haverford Alchemy
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Reactions of Aromatic Compounds
... that is more deactivating than halogens. Rearrangements are possible. The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. ...
... that is more deactivating than halogens. Rearrangements are possible. The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. ...
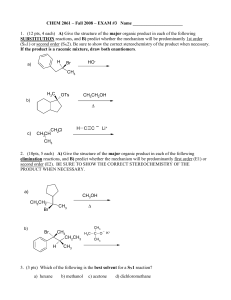
Exam #3
... reactions. Clearly indicate stereochemistry where appropriate. If the major product is a pair of enantiomers, only draw one of the two structures. ...
... reactions. Clearly indicate stereochemistry where appropriate. If the major product is a pair of enantiomers, only draw one of the two structures. ...
Tunge - IARC Research
... seven-membered (98 %). Similar variation of enolate structure while keeping the allyl fragment constant (cyclohexenyl) shows that a variety of enolates add with selectivities that are relatively independent of steric size. While most substrates were rearranged in the presence of 5 mol % Pd2dba3 and ...
... seven-membered (98 %). Similar variation of enolate structure while keeping the allyl fragment constant (cyclohexenyl) shows that a variety of enolates add with selectivities that are relatively independent of steric size. While most substrates were rearranged in the presence of 5 mol % Pd2dba3 and ...
Power Point for Equilibrium
... • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to the rate of the reverse reac ...
... • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to the rate of the reverse reac ...
Paper I
... Only scattered information about the mechanism of dehydrogenation is available for chromium on alumina and none at all for silica. Starting with various ideas about the nature of the active site, a number of reaction mechanisms have been proposed, see Ref. 36 and references cited therein. A recurren ...
... Only scattered information about the mechanism of dehydrogenation is available for chromium on alumina and none at all for silica. Starting with various ideas about the nature of the active site, a number of reaction mechanisms have been proposed, see Ref. 36 and references cited therein. A recurren ...
Disproportionation of Monolithium Acetylide into
... most widely used ethynylation and alkynylation reaction.1 Monolithium acetylide (1) disproportionates readily above -25 °C into the more stable dilithium carbide (2) and acetylene.2 Liquid ammonia which is utilized in processes claimed to have industrial economics1,3 serves not only as a solvent but ...
... most widely used ethynylation and alkynylation reaction.1 Monolithium acetylide (1) disproportionates readily above -25 °C into the more stable dilithium carbide (2) and acetylene.2 Liquid ammonia which is utilized in processes claimed to have industrial economics1,3 serves not only as a solvent but ...
Grignard Reaction - OpenBU
... dry ice has sublimed. The reaction mixture should be a viscous syrup or frothy solid. Workup: Slowly add 10 mL 6.0 M HCl (aq.) to the beaker with swirling. Any remaining magnesium will react to form hydrogen gas. If you still have solid remaining, add a little more HCl solution or more diethyl ether ...
... dry ice has sublimed. The reaction mixture should be a viscous syrup or frothy solid. Workup: Slowly add 10 mL 6.0 M HCl (aq.) to the beaker with swirling. Any remaining magnesium will react to form hydrogen gas. If you still have solid remaining, add a little more HCl solution or more diethyl ether ...
Grignard Reaction - OpenBU
... dry ice has sublimed. The reaction mixture should be a viscous syrup or frothy solid. Workup: Slowly add 10 mL 6.0 M HCl (aq.) to the beaker with swirling. Any remaining magnesium will react to form hydrogen gas. If you still have solid remaining, add a little more HCl solution or more diethyl ether ...
... dry ice has sublimed. The reaction mixture should be a viscous syrup or frothy solid. Workup: Slowly add 10 mL 6.0 M HCl (aq.) to the beaker with swirling. Any remaining magnesium will react to form hydrogen gas. If you still have solid remaining, add a little more HCl solution or more diethyl ether ...
Sustainable Oxidation Catalysis for Synthesis
... * corresponding author: [email protected] Abstract Conventional wastewater treatment plants are not effective when wastewaters have to be treated due to the high COD load and the presence of recalcitrant compounds. An alternative to conventional water treatments are Advanced Oxidation Processes (A ...
... * corresponding author: [email protected] Abstract Conventional wastewater treatment plants are not effective when wastewaters have to be treated due to the high COD load and the presence of recalcitrant compounds. An alternative to conventional water treatments are Advanced Oxidation Processes (A ...
07. Aldehydes and ketones
... As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
... As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.