interaction of alcohols with alkalies under autogeneous pressure
... of sodium hydroxide taken. Reid, Worthington and Larchar 4 on the other hand have claimed an yield of 60-95% by working at a pressure of 400 atmospheres and at 320° C. Stephenson and Pelton 5 have patented processes involving the reaction of alkali with a number of alcohols. Stochiometric relationsh ...
... of sodium hydroxide taken. Reid, Worthington and Larchar 4 on the other hand have claimed an yield of 60-95% by working at a pressure of 400 atmospheres and at 320° C. Stephenson and Pelton 5 have patented processes involving the reaction of alkali with a number of alcohols. Stochiometric relationsh ...
Metal-Catalyzed Epoxidations of Alkenes with Hydrogen
... does not undergo redox chemistry during catalytic reactions; it remains in the +VII oxidation state. MTO activates H2O2 through an equilibrium formation of the η2-peroxo species 8 and 9. The structure of 9 was confirmed crystallographically, and the methyl resonances of 8 and 9 were detected by both ...
... does not undergo redox chemistry during catalytic reactions; it remains in the +VII oxidation state. MTO activates H2O2 through an equilibrium formation of the η2-peroxo species 8 and 9. The structure of 9 was confirmed crystallographically, and the methyl resonances of 8 and 9 were detected by both ...
Learning Guide for Chapter 9 - Alkyl Halides I
... high polarity solvent - to dissolve charged reagents aprotic solvent - hydrogen bonding slows down the reaction surrounds Nu/base, makes it hard to react What kind of solvent do SN1 reactions require? polarity isn't important - reagents are not charged, dissolve easily protic solvent - stabilizes th ...
... high polarity solvent - to dissolve charged reagents aprotic solvent - hydrogen bonding slows down the reaction surrounds Nu/base, makes it hard to react What kind of solvent do SN1 reactions require? polarity isn't important - reagents are not charged, dissolve easily protic solvent - stabilizes th ...
VITA - Trace: Tennessee Research and Creative Exchange
... case by Hillhouse it was found that although the starting Ni-complex would not activate an organic azide, a Ni-imido could be prepared synthetically (Figure 1.1B) and the nitrene does transfer to an alkene to form an aziridine. 10c Still the lack of a system which can combine both the activation ste ...
... case by Hillhouse it was found that although the starting Ni-complex would not activate an organic azide, a Ni-imido could be prepared synthetically (Figure 1.1B) and the nitrene does transfer to an alkene to form an aziridine. 10c Still the lack of a system which can combine both the activation ste ...
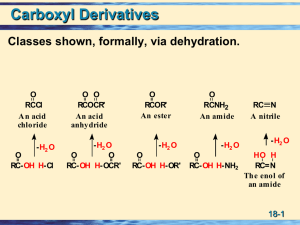
carboxylic acid
... Acyl adenylate is a mixed anhydride between carboxylic and adenosine monophosphate (AMP), also known as adenylic acid Occurs in biosynthesis of fats ...
... Acyl adenylate is a mixed anhydride between carboxylic and adenosine monophosphate (AMP), also known as adenylic acid Occurs in biosynthesis of fats ...
Document
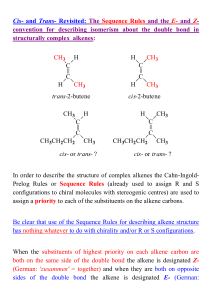
... • When a mixture of stereoisomers is possible from a dehydrohalogenation, the major product is the more stable stereoisomer. • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one ste ...
... • When a mixture of stereoisomers is possible from a dehydrohalogenation, the major product is the more stable stereoisomer. • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one ste ...
Novel Transition Metal-Catalysed Syntheses of Carboxylic Acid
... for two or more transformations that are fundamentally different in nature.[13] This area is one of great potential since there are many catalytic reactions which potentially can be linked in sequence. The first reported reactions of this nature were sequential transformations performed by a single ...
... for two or more transformations that are fundamentally different in nature.[13] This area is one of great potential since there are many catalytic reactions which potentially can be linked in sequence. The first reported reactions of this nature were sequential transformations performed by a single ...
nucleophilic addition on ketones and ketimines - ISI
... The asymmetric allylation of ketones was also recently studied by the groups of Shibasaki22 and Yamamoto23 using soft transition metal–chiral diphosphine complexes as catalysts (Scheme 9). Shibasaki et al. used allyl boronates as nucleophiles for the allyl transfer on aliphatic and aromatic ketones ...
... The asymmetric allylation of ketones was also recently studied by the groups of Shibasaki22 and Yamamoto23 using soft transition metal–chiral diphosphine complexes as catalysts (Scheme 9). Shibasaki et al. used allyl boronates as nucleophiles for the allyl transfer on aliphatic and aromatic ketones ...
Organic Chemistry
... hydride (DIBAlH) at -78°C selectively reduces an ester to an aldehyde. • At -78°C, the TCAI does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated. O OCH3 Methyl hexanoate ...
... hydride (DIBAlH) at -78°C selectively reduces an ester to an aldehyde. • At -78°C, the TCAI does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated. O OCH3 Methyl hexanoate ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.