CHM 222 - Jefferson State Community College
... Demonstrate an understanding of reactions involving aldehydes, ketones, carboxylic acids and their derivatives including nomenclature, synthesis and mechanisms. 1. Name and draw aldehydes, ketones, carboxylic acids and their derivatives. 2. Propose a synthesis for each type of compound listed above. ...
... Demonstrate an understanding of reactions involving aldehydes, ketones, carboxylic acids and their derivatives including nomenclature, synthesis and mechanisms. 1. Name and draw aldehydes, ketones, carboxylic acids and their derivatives. 2. Propose a synthesis for each type of compound listed above. ...
Formation of Acetic Acid by Aqueous-Phase Oxidation
... and O2 pressures of 0.6 MPa. This reaction proceeds readily in aqueous acidic media and yields of up to 90 % are achieved, with CO2 as the only major by-product. Thus, it constitutes a very simple, green route to acetic acid. The oxidation of ethanol by air into acetic acid over platinum was among t ...
... and O2 pressures of 0.6 MPa. This reaction proceeds readily in aqueous acidic media and yields of up to 90 % are achieved, with CO2 as the only major by-product. Thus, it constitutes a very simple, green route to acetic acid. The oxidation of ethanol by air into acetic acid over platinum was among t ...
Practice Questions - Elevate Education
... its chains. Thermosets have permanent cross-links (covalent bonds). (OR it is recyclable and permanent cross-links prevent recycling) ...
... its chains. Thermosets have permanent cross-links (covalent bonds). (OR it is recyclable and permanent cross-links prevent recycling) ...
Experiment 4- Alkene
... (ii) Reactions of Alkenes Alkenes, containing a site of unsaturation, undergo electrophilic addition reactions with several reagents such as halogens, oxidizing agents, and sulfuric, halogen, and hypohalous acids. In particular, bromine and oxidizing agents such as permanganate are widely used in qu ...
... (ii) Reactions of Alkenes Alkenes, containing a site of unsaturation, undergo electrophilic addition reactions with several reagents such as halogens, oxidizing agents, and sulfuric, halogen, and hypohalous acids. In particular, bromine and oxidizing agents such as permanganate are widely used in qu ...
Week 10 Problem Set (Answers) (4/17, 4/18, 4/19) Reactions and
... it to be symmetrical, the ring itself should be symmetrical. Upon being subjected to oxymercuration-reduction conditions, Compound B yields at least one chiral compound. This is untrue for Compound D. Now you can separate the two possible cyclopropanes you came up with earlier for B and D. Upon reac ...
... it to be symmetrical, the ring itself should be symmetrical. Upon being subjected to oxymercuration-reduction conditions, Compound B yields at least one chiral compound. This is untrue for Compound D. Now you can separate the two possible cyclopropanes you came up with earlier for B and D. Upon reac ...
rev2
... e. Oxidation with chromic acid or KMnO4. A primary alcohol goes to aldehyde and secondary goes to ketone. Tertiary does not oxidize. 7. Know about the structure of phenols. 8. Be able to name phenols 9. Phenols are more soluble in water than alcohols because they are more polar. Know about the chemi ...
... e. Oxidation with chromic acid or KMnO4. A primary alcohol goes to aldehyde and secondary goes to ketone. Tertiary does not oxidize. 7. Know about the structure of phenols. 8. Be able to name phenols 9. Phenols are more soluble in water than alcohols because they are more polar. Know about the chemi ...
Chapter 20 reactions of carbonyls
... • CBS refers to Corey, Bakshi, and Shibata, the chemists who developed these versatile reagents. • One B–H bond serves as the source of hydride in this reduction. • The (S)-CBS reagent delivers (H:−) from the front side of the C=O. This generally affords the R alcohol as the major product. • The (R) ...
... • CBS refers to Corey, Bakshi, and Shibata, the chemists who developed these versatile reagents. • One B–H bond serves as the source of hydride in this reduction. • The (S)-CBS reagent delivers (H:−) from the front side of the C=O. This generally affords the R alcohol as the major product. • The (R) ...
Ch 23 Carbonyl Condensations
... - The -hydroxyl can be removed along with an H to create a bond. The result is a conjugated enone (or enal). - This also drives the aldol reaction’s equilibrium towards the products. - The dehydration can be catalyzed either by acid or base, along with mild heating. - The base-catalyzed reactio ...
... - The -hydroxyl can be removed along with an H to create a bond. The result is a conjugated enone (or enal). - This also drives the aldol reaction’s equilibrium towards the products. - The dehydration can be catalyzed either by acid or base, along with mild heating. - The base-catalyzed reactio ...
Experimen tt: Dehydration of an Alcohol
... E1 mechanism in which water is lost, forming a carbocation. This carbocation, in turn, loses a proton to form the alkene product. Tertiary alcohols undergo dehydration most easily because they form more stable carbocations. Because carbocations can rearrange to form equally stable or more stable car ...
... E1 mechanism in which water is lost, forming a carbocation. This carbocation, in turn, loses a proton to form the alkene product. Tertiary alcohols undergo dehydration most easily because they form more stable carbocations. Because carbocations can rearrange to form equally stable or more stable car ...
Document
... anti-cancer reagent. Developed a new type of non-toxic compounds, which are highly effective for curing theschistosomiasis. 2. RNA and DNA cleavage reagents Discovered that the N-phospho-histidine could cleave RNA and DNA by hydrolysis on the phospho-diester bond, Also found that N-phospho-serine i ...
... anti-cancer reagent. Developed a new type of non-toxic compounds, which are highly effective for curing theschistosomiasis. 2. RNA and DNA cleavage reagents Discovered that the N-phospho-histidine could cleave RNA and DNA by hydrolysis on the phospho-diester bond, Also found that N-phospho-serine i ...
aldehydes powerpoint
... group is always number 1. 3) Identify the branched attachments (alphabetically) and prefix the carbon number it is attached to. If there is more than one of the same type use prefixes. Ex: di for 2, tri for 3 ...
... group is always number 1. 3) Identify the branched attachments (alphabetically) and prefix the carbon number it is attached to. If there is more than one of the same type use prefixes. Ex: di for 2, tri for 3 ...
CN>Chapter 22CT>Carbonyl Alpha
... as being in the α position Electrophilic substitution occurs at this position through either an enol or enolate ion ...
... as being in the α position Electrophilic substitution occurs at this position through either an enol or enolate ion ...
Chemistry Definitions by Units
... exhibits optical activity. A chiral center is usually but not necessarily present. Cis isomers: Configuration about a double bond in which the two largest groups are on the same side of the molecule. (See Geometric isomers). Condensation reaction: Combination of two or more molecules, often with the ...
... exhibits optical activity. A chiral center is usually but not necessarily present. Cis isomers: Configuration about a double bond in which the two largest groups are on the same side of the molecule. (See Geometric isomers). Condensation reaction: Combination of two or more molecules, often with the ...
ORGSEQPP.pps
... It may be surprising that water needs such vigorous conditions to react with ethene. It is a highly polar molecule and you would expect it to be a good electrophile. ...
... It may be surprising that water needs such vigorous conditions to react with ethene. It is a highly polar molecule and you would expect it to be a good electrophile. ...
C 2 H 5 OH(l)
... It may be surprising that water needs such vigorous conditions to react with ethene. It is a highly polar molecule and you would expect it to be a good electrophile. ...
... It may be surprising that water needs such vigorous conditions to react with ethene. It is a highly polar molecule and you would expect it to be a good electrophile. ...
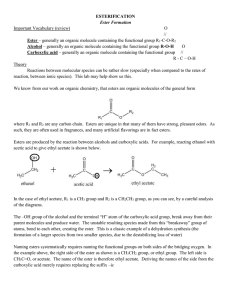
ESTERIFICATION Ester Formation Important Vocabulary (review) O
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
... In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH2CH3 group, as you can see, by a careful analysis of the diagrams. The –OH group of the alcohol and the terminal “H” atom of the carboxylic acid group, break away from their parent molecules and produce water. The unstable resulting speci ...
Experiment #3: Asymmetric Synthesis – Use of a Chiral Manganese
... (Note: The addition of the diamine is exothermic.) The solution is initially cloudy, but complete dissolution is observed within min. After dissolution is complete, 5.0 mL of glacial acetic acid is added in one portion. Product begins to precipitate from solution shortly after the addition, and it c ...
... (Note: The addition of the diamine is exothermic.) The solution is initially cloudy, but complete dissolution is observed within min. After dissolution is complete, 5.0 mL of glacial acetic acid is added in one portion. Product begins to precipitate from solution shortly after the addition, and it c ...
Exp`t 73
... Dehydration of 2-methylcyclohexanol, 1 (B.P. = 163-166°C) with 85% phosphoric acid yields a mixture of three products: the main product overall (75-80%) is 1-methyl-1-cyclohexene, 2 (B.P. = 110-111 °C); also present are 3-methyl-1-cyclohexene, 3 (B.P.=104 °C) and methylenecyclohexane, 4. The relativ ...
... Dehydration of 2-methylcyclohexanol, 1 (B.P. = 163-166°C) with 85% phosphoric acid yields a mixture of three products: the main product overall (75-80%) is 1-methyl-1-cyclohexene, 2 (B.P. = 110-111 °C); also present are 3-methyl-1-cyclohexene, 3 (B.P.=104 °C) and methylenecyclohexane, 4. The relativ ...
... with hydrophobic hydrocarbon chain. They have good emulsification activity and wetting properties. Furthermore, the sugar head group could ionize with no charge. Thus, they have potential use as effective agents in membrane solubilization and also in food and cosmetic industries [1]. Enzymatic synth ...
PARA-AMINOBENZOIC ACID (PABA) Functions of
... May prevent amines from forming hallucinogens; used in schizophrenia (2,000 mg/day); With folic acid, B-5 & biotin, PABA restores colour to grey & greying hair (animals); Used to treat Peyronie’s disease, a fibrous penis condition in post-middle aged men; Used in lupus, apparently with some success; ...
... May prevent amines from forming hallucinogens; used in schizophrenia (2,000 mg/day); With folic acid, B-5 & biotin, PABA restores colour to grey & greying hair (animals); Used to treat Peyronie’s disease, a fibrous penis condition in post-middle aged men; Used in lupus, apparently with some success; ...
Carboxylic Acid Derivatives 1. Background and Properties
... Carboxylic Acid Derivatives 1. Background and Properties The important classes of organic compounds known as alcohols, phenols, ethers, amines and halides consist of alkyl and/or aryl groups bonded to hydroxyl, alkoxyl, amino and halo substituents respectively. If these same functional groups are at ...
... Carboxylic Acid Derivatives 1. Background and Properties The important classes of organic compounds known as alcohols, phenols, ethers, amines and halides consist of alkyl and/or aryl groups bonded to hydroxyl, alkoxyl, amino and halo substituents respectively. If these same functional groups are at ...
Organic Chemistry I Laboratory
... isolated. Because only a very small percentage of the menthyl chloride molecules are in the less stable conformation at any time, the reaction is much slower than the reaction of neomenthyl chloride. This example shows that whenever a substrate yields the less stable alkene as a major product of an ...
... isolated. Because only a very small percentage of the menthyl chloride molecules are in the less stable conformation at any time, the reaction is much slower than the reaction of neomenthyl chloride. This example shows that whenever a substrate yields the less stable alkene as a major product of an ...
ADDITION REACTIONS
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.