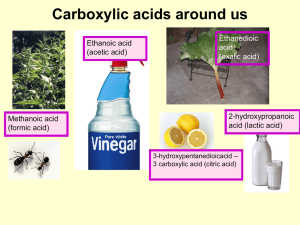
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... What will be the major product formed on dehydrohalogenation of 2-bromo-2,3dimethylbutane. 06. Name the following alcohols by carbinol and IUPAC system: (i) CH3CHOHCH=CH2 (ii) CH3CH2C(CH3)(OH)CH2CH2CH3 07. COC bond angle in diethyl ether is greater than HOH bond angle in water. Explain. 0 ...
... What will be the major product formed on dehydrohalogenation of 2-bromo-2,3dimethylbutane. 06. Name the following alcohols by carbinol and IUPAC system: (i) CH3CHOHCH=CH2 (ii) CH3CH2C(CH3)(OH)CH2CH2CH3 07. COC bond angle in diethyl ether is greater than HOH bond angle in water. Explain. 0 ...
worksheet Ka Kb buffers Ksp
... b. If a reaction occurs, how fast will it occur? c. What is the mechanism by which the reaction occurs? d. If substances react, what energy changes are associated with the reaction? ...
... b. If a reaction occurs, how fast will it occur? c. What is the mechanism by which the reaction occurs? d. If substances react, what energy changes are associated with the reaction? ...
amine
... • Name the longest chain attached to the nitrogen. • Replace the final –e with –amine. • Number the chain so the carbon bonded to the nitrogen has the lowest possible number. • Number the other substituents on the carbon chain. • An italic “N” is used as a prefix for a substituent on nitrogen. Examp ...
... • Name the longest chain attached to the nitrogen. • Replace the final –e with –amine. • Number the chain so the carbon bonded to the nitrogen has the lowest possible number. • Number the other substituents on the carbon chain. • An italic “N” is used as a prefix for a substituent on nitrogen. Examp ...
Aromatic amines The
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
... A Nitrogen, containing a lone pair is the key atom Resembles Ammonia where one or more H’s Are replaced by alkyl groups The lone pair participates in the reactivity Of amines Amines are a core part of ‘amino acids’ ...
File
... 10. Draw the Lewis structures for each of the following molecules or ions. 2pt each a. H2S b. SO2 c. PO3-3 ...
... 10. Draw the Lewis structures for each of the following molecules or ions. 2pt each a. H2S b. SO2 c. PO3-3 ...
Review and New - ChemConnections
... • Treatment of 1-propanol with K2Cr2O7, H2SO4, and heat will produce: ...
... • Treatment of 1-propanol with K2Cr2O7, H2SO4, and heat will produce: ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 01. What are the effects of reagents and solvents in Stobbe reaction? Give examples. 02. How is carbene synthesized? In what form it is used in reactions? Give an example. 03. Identify any two types of 1,3-dipolar compounds used for cycloaddition reactions. Give an example. ...
... 01. What are the effects of reagents and solvents in Stobbe reaction? Give examples. 02. How is carbene synthesized? In what form it is used in reactions? Give an example. 03. Identify any two types of 1,3-dipolar compounds used for cycloaddition reactions. Give an example. ...
A Floral Fragrance, Methyl Benzoate
... Isolation and Purification Cool the solution to room temperature, then decant it into a separatory funnel containing 25 mL of water, and rinse the flask with 25 mL of diethyl ether (use wet ether found in a supply bottle in each hood). Add this ether to the separatory funnel, shake thoroughly, and ...
... Isolation and Purification Cool the solution to room temperature, then decant it into a separatory funnel containing 25 mL of water, and rinse the flask with 25 mL of diethyl ether (use wet ether found in a supply bottle in each hood). Add this ether to the separatory funnel, shake thoroughly, and ...
Applications of titrimetry
... • Usually triggered by a (relatively) large change in a property of the solution - change in pH of solution - Demf or Dcurrent flow in a solution ...
... • Usually triggered by a (relatively) large change in a property of the solution - change in pH of solution - Demf or Dcurrent flow in a solution ...
Chem 231 Exam #1 Study Guide
... Know how to draw (without models) different conformations of ethane and butane (eclipsed, staggered) and predict their energies Know how to draw Newman projections as well as wedge and dash drawings Know how to draw (without models) different conformations of cyclohexane Be able to distinguish betwe ...
... Know how to draw (without models) different conformations of ethane and butane (eclipsed, staggered) and predict their energies Know how to draw Newman projections as well as wedge and dash drawings Know how to draw (without models) different conformations of cyclohexane Be able to distinguish betwe ...
L1 - Amines
... • Organic bases. • Generally have strong, unpleasant odors. • Are found extensively in biological systems. • Found in both controlled and medicinal compounds 1,5 - diaminopentane H2N – CH2-CH2-CH2-CH2-CH2 – NH2 Cadaverine ...
... • Organic bases. • Generally have strong, unpleasant odors. • Are found extensively in biological systems. • Found in both controlled and medicinal compounds 1,5 - diaminopentane H2N – CH2-CH2-CH2-CH2-CH2 – NH2 Cadaverine ...
CHEMISTRY 1000
... chlorides (since the pKa values for HCl and RSO3H are about -7). Therefore, if converting the alcohol into a sulfonate ester is helpful, it is reasonable to conclude that converting the alcohol into the corresponding alkyl halide would be equally helpful. ...
... chlorides (since the pKa values for HCl and RSO3H are about -7). Therefore, if converting the alcohol into a sulfonate ester is helpful, it is reasonable to conclude that converting the alcohol into the corresponding alkyl halide would be equally helpful. ...
Amines - ChemConnections
... reabsorption of the drug through the renal system.[11] Co-administration of acidic substances (e.g. citric acid) causes decreased renal reabsorption of DL-amphetamine, while alkaline agents (e.g. antacids) may cause a marked increase in renal tubular reabsorption. The increased reabsorption can incr ...
... reabsorption of the drug through the renal system.[11] Co-administration of acidic substances (e.g. citric acid) causes decreased renal reabsorption of DL-amphetamine, while alkaline agents (e.g. antacids) may cause a marked increase in renal tubular reabsorption. The increased reabsorption can incr ...
CfE Higher Chemistry Unit 2: Nature‟s Chemistry Esters, Fats and
... D liquid at room temperature and contain a high proportion of saturated molecules. ...
... D liquid at room temperature and contain a high proportion of saturated molecules. ...
PPT
... • Replace the final –e with –amine. • Number the chain so the carbon bonded to the nitrogen has the lowest possible number. • Number the other substituents on the carbon chain. • An italic “N” is used as a prefix for a substituent on nitrogen. Examples: ...
... • Replace the final –e with –amine. • Number the chain so the carbon bonded to the nitrogen has the lowest possible number. • Number the other substituents on the carbon chain. • An italic “N” is used as a prefix for a substituent on nitrogen. Examples: ...
Nugget
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.