Exam 1 from 2008
... a) Draw an accurate 3D structure of allene. (Hint: It is not planar.) On this structure show the p-orbitals and how they overlap to form the pi bonds in allene. Clearly indicate the 3D orientation of the p-orbitals relative to the atoms in the molecule and to each other. b) Label the hybridization o ...
... a) Draw an accurate 3D structure of allene. (Hint: It is not planar.) On this structure show the p-orbitals and how they overlap to form the pi bonds in allene. Clearly indicate the 3D orientation of the p-orbitals relative to the atoms in the molecule and to each other. b) Label the hybridization o ...
Chem 263 April 11, 2006 Reductive Amination Amines can be
... Nicotine is found naturally in tobacco and is shown below. It constitutes 0.3 to 5% of the tobacco plant by dry weight. It is a potent nerve poison and is included in many insecticides. In lower concentrations, the substance is a stimulant and is one of the main factors leading to the habit-forming ...
... Nicotine is found naturally in tobacco and is shown below. It constitutes 0.3 to 5% of the tobacco plant by dry weight. It is a potent nerve poison and is included in many insecticides. In lower concentrations, the substance is a stimulant and is one of the main factors leading to the habit-forming ...
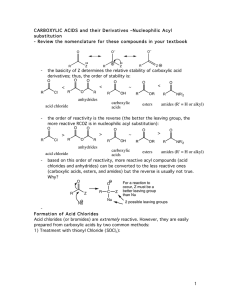
carboxylic acids esters amides (R
... to prepare in bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl). Second, cyclic anhydrides are fairly easily formed by heating molecules that have two carboxylic acid functions in close proximity to high temperatures (a dehydration reaction). A couple of ...
... to prepare in bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl). Second, cyclic anhydrides are fairly easily formed by heating molecules that have two carboxylic acid functions in close proximity to high temperatures (a dehydration reaction). A couple of ...
Edexcel GCE - The Student Room
... Information for Candidates A Periodic Table is printed on the back cover of this paper. The marks for individual questions and the parts of questions are shown in round brackets: e.g. (2). The total mark for this paper is 60. There are 16 pages in this paper. Any blank pages are indicated. ...
... Information for Candidates A Periodic Table is printed on the back cover of this paper. The marks for individual questions and the parts of questions are shown in round brackets: e.g. (2). The total mark for this paper is 60. There are 16 pages in this paper. Any blank pages are indicated. ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry
... 14. Cooking changes the appearance and composition of food. Using your knowledge of chemistry, comment on the changes to food which may occur during cooking. ...
... 14. Cooking changes the appearance and composition of food. Using your knowledge of chemistry, comment on the changes to food which may occur during cooking. ...
Exp 19 - Diphenylacetylene_2015
... 1. Bromination of trans-Stilbene (Preparation of meso-Stilbene Dibromide) Add approximately 180 mg of trans-stilbene (MW = 180 g/mol) and 2.0 mL of dichloromethane (DCM) to a 5 mL conical vial containing a spin vane. Place the vial on the stir-plate in the aluminum block and stir until the stilbene ...
... 1. Bromination of trans-Stilbene (Preparation of meso-Stilbene Dibromide) Add approximately 180 mg of trans-stilbene (MW = 180 g/mol) and 2.0 mL of dichloromethane (DCM) to a 5 mL conical vial containing a spin vane. Place the vial on the stir-plate in the aluminum block and stir until the stilbene ...
Chem 231 Exam #1 Study Guide
... Be able to fill in missing reactants, products or catalysts for hydrogenation of alkenes and alkynes. Look at the general reaction. Be able to fill in missing reactants, products or catalysts for the reduction of alkyl halides. Look at the general reaction. Be able to fill in missing reactants, prod ...
... Be able to fill in missing reactants, products or catalysts for hydrogenation of alkenes and alkynes. Look at the general reaction. Be able to fill in missing reactants, products or catalysts for the reduction of alkyl halides. Look at the general reaction. Be able to fill in missing reactants, prod ...
ACP Chemistry Semester 1 Final Exam - Doc-U-Ment
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
Organic Reactions
... Ability to distinguish reactants and products in a reaction Naming and identifying organic compounds Ability to distinguishing between saturated and unsaturated hydrocarbons Naming and recognizing functional groups in organic compounds ...
... Ability to distinguish reactants and products in a reaction Naming and identifying organic compounds Ability to distinguishing between saturated and unsaturated hydrocarbons Naming and recognizing functional groups in organic compounds ...
Practice Paper - 3
... What are antibiotics? Distinguish between narrow spectrum and broad spectrum antibiotics. Clasify the following into Bactericidal and Bacteriostatic antibiotics. An organic compound A (C3H6O) is resistant to oxidation but form compound B (C3H8O) on reduction B reacts with HBr to form the compound C. ...
... What are antibiotics? Distinguish between narrow spectrum and broad spectrum antibiotics. Clasify the following into Bactericidal and Bacteriostatic antibiotics. An organic compound A (C3H6O) is resistant to oxidation but form compound B (C3H8O) on reduction B reacts with HBr to form the compound C. ...
Lecture 18
... medulla during stressful situations They raise the blood glucose level and move blood to the muscles. The prefix nor in a drug name means there is one less —CH3 group on the nitrogen atom. Norepinephrine is used in remedies for colds, hay fever, and asthma because it contracts the capillaries in the ...
... medulla during stressful situations They raise the blood glucose level and move blood to the muscles. The prefix nor in a drug name means there is one less —CH3 group on the nitrogen atom. Norepinephrine is used in remedies for colds, hay fever, and asthma because it contracts the capillaries in the ...
Seminar_1 1. Classification and nomenclature of organic
... strain. The smallest cycloalkane is cyclopropane, which forms a triangular molecule which is much more reactive than straight–chain propane. Cyclobutane forms a square molecule, which is less reactive than cyclopropane, but is more reactive than butane. Cyclopentane and higher cycloalkanes have a si ...
... strain. The smallest cycloalkane is cyclopropane, which forms a triangular molecule which is much more reactive than straight–chain propane. Cyclobutane forms a square molecule, which is less reactive than cyclopropane, but is more reactive than butane. Cyclopentane and higher cycloalkanes have a si ...
Topic 11 Organic Chemistry
... 2. This question is about four compounds A, B, C and D, which can be made from ethene by the following reactions. All four compounds are liquid at room temperature, and each compound's molecular formula is shown. ...
... 2. This question is about four compounds A, B, C and D, which can be made from ethene by the following reactions. All four compounds are liquid at room temperature, and each compound's molecular formula is shown. ...
Biochemistry 462a - Enzymes Extra Questions
... chain groups of the protein are titrated? Explain. 5. Explain the following observation. A rule of thumb is that a chemical reaction will go twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming ...
... chain groups of the protein are titrated? Explain. 5. Explain the following observation. A rule of thumb is that a chemical reaction will go twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming ...
ENZYMES - SELF STUDY QUESTIONS 1. A chemical reaction has a
... chain groups of the protein are titrated? Explain. 5. Explain the following observation. A rule of thumb is that a chemical reaction will go twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming ...
... chain groups of the protein are titrated? Explain. 5. Explain the following observation. A rule of thumb is that a chemical reaction will go twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming ...
슬라이드 1
... Isotope effects indicate that the collapse of the adduct by reductive elimination is the rate determining step. The more easily reduced, the more reactive is the compound toward cuprate reagents. Compounds such as a,b-unsaturated esters and nitriles, which are not as easily reduced as the correspond ...
... Isotope effects indicate that the collapse of the adduct by reductive elimination is the rate determining step. The more easily reduced, the more reactive is the compound toward cuprate reagents. Compounds such as a,b-unsaturated esters and nitriles, which are not as easily reduced as the correspond ...
CHEMISTRY 1000
... chlorides (since the pKa values for HCl and RSO3H are about -7). Therefore, if converting the alcohol into a sulfonate ester is helpful, it is reasonable to conclude that converting the alcohol into the corresponding alkyl halide would be equally helpful. ...
... chlorides (since the pKa values for HCl and RSO3H are about -7). Therefore, if converting the alcohol into a sulfonate ester is helpful, it is reasonable to conclude that converting the alcohol into the corresponding alkyl halide would be equally helpful. ...
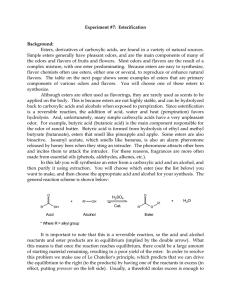
102 Lab 7 Esters Fall05
... alcohol or the acid can be used in excess. The choice can be based on cost, availability and/or ease of purification at the end of the reaction. In this reaction we will add an excess of the acid. Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which ...
... alcohol or the acid can be used in excess. The choice can be based on cost, availability and/or ease of purification at the end of the reaction. In this reaction we will add an excess of the acid. Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which ...
enzymatic resolution of a racemic mixture by acylation in
... Biocatalysis is a convenient method for the kinetic resolution of alcohols. There are many reports in the literature on the resolution of secondary alcohols in ionic liquids [3], [4], [5-8]. Of these, only a few refer to aliphatic alcohols, in particular of longer alkyl chain lengths [3], [5], [8]. ...
... Biocatalysis is a convenient method for the kinetic resolution of alcohols. There are many reports in the literature on the resolution of secondary alcohols in ionic liquids [3], [4], [5-8]. Of these, only a few refer to aliphatic alcohols, in particular of longer alkyl chain lengths [3], [5], [8]. ...
Chapter 7
... • The slow step, RDS, is the second step, the formation of the carbocation • This explains the order of reactivity with the tertiary alcohol reacting easiest, due to the tertiary carbocation being the most stable. ...
... • The slow step, RDS, is the second step, the formation of the carbocation • This explains the order of reactivity with the tertiary alcohol reacting easiest, due to the tertiary carbocation being the most stable. ...
Practice Test 2
... Vinegar contains a weak acid, acetic acid (HC2H3O2), which is responsible for its acidity. In one analysis of a commercial vinegar brand, a 15.0 mL sample was titrated with 0.4500 M NaOH. It required 30.50 mL of this NaOH solution to neutralize the acid in the vinegar sample. What is the molar conce ...
... Vinegar contains a weak acid, acetic acid (HC2H3O2), which is responsible for its acidity. In one analysis of a commercial vinegar brand, a 15.0 mL sample was titrated with 0.4500 M NaOH. It required 30.50 mL of this NaOH solution to neutralize the acid in the vinegar sample. What is the molar conce ...
Organic Pathways
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
Second Semester Final Review Guide
... 4. The substance containing the element that causes another element to gain electrons is called what? a. Oxidizing agent ...
... 4. The substance containing the element that causes another element to gain electrons is called what? a. Oxidizing agent ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.