Word Version of Answer Key
... belongs to a class of compounds called alcohols (which is why we call drinks with this type of molecule alcohol). All alcohols have a hydroxide molecule (-OH) attached to one of the carbon atoms in the molecule, and it is this chemical makeup that makes the breakdown of alcohol so much different fro ...
... belongs to a class of compounds called alcohols (which is why we call drinks with this type of molecule alcohol). All alcohols have a hydroxide molecule (-OH) attached to one of the carbon atoms in the molecule, and it is this chemical makeup that makes the breakdown of alcohol so much different fro ...
the chemistry of smell
... Repeat this process with your other assigned ester making sure to label the test tube. 4. Place the test tubes in the 80-85C water bath, using test tube holders to keep the tubes from floating or tipping in the beaker. Heat for about 20 minutes mixing the solution from time to time and maintaining ...
... Repeat this process with your other assigned ester making sure to label the test tube. 4. Place the test tubes in the 80-85C water bath, using test tube holders to keep the tubes from floating or tipping in the beaker. Heat for about 20 minutes mixing the solution from time to time and maintaining ...
Lecture
... humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism and therefore must be part of its diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis. ...
... humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism and therefore must be part of its diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis. ...
Grade 11: Physical Sciences Outline
... Condensed structural formula: This notation shows the way in which atoms are bonded together in the molecule, but DOES NOT SHOW ALL bond lines. Hydrocarbon: Organic compounds that consist of hydrogen and carbon only. Homologous series: A series of organic compounds that can be described by the same ...
... Condensed structural formula: This notation shows the way in which atoms are bonded together in the molecule, but DOES NOT SHOW ALL bond lines. Hydrocarbon: Organic compounds that consist of hydrogen and carbon only. Homologous series: A series of organic compounds that can be described by the same ...
How is Alcohol Metabolized?
... belongs to a class of compounds called alcohols (which is why we call drinks with this type of molecule alcohol). All alcohols have a hydroxide molecule (-OH) attached to one of the carbon atoms in the molecule, and it is this chemical makeup that makes the breakdown of alcohol so much different fro ...
... belongs to a class of compounds called alcohols (which is why we call drinks with this type of molecule alcohol). All alcohols have a hydroxide molecule (-OH) attached to one of the carbon atoms in the molecule, and it is this chemical makeup that makes the breakdown of alcohol so much different fro ...
A-level Paper 3 Practice Paper 3 - A
... This question is about a method that can be used to prepare ethylamine. CH3CH2Br + 2NH3 ...
... This question is about a method that can be used to prepare ethylamine. CH3CH2Br + 2NH3 ...
Monosaccharide
... • Because the anomeric carbon atom is chiral, two possible stereoisomers can be formed during cyclization. • An anomer (-OH on the anomeric carbon pointing down) • A anomer (-OH on the anomeric carbon pointing up) • Anomers are stereoisomers that differ in the 3-D arrangement of groups at the an ...
... • Because the anomeric carbon atom is chiral, two possible stereoisomers can be formed during cyclization. • An anomer (-OH on the anomeric carbon pointing down) • A anomer (-OH on the anomeric carbon pointing up) • Anomers are stereoisomers that differ in the 3-D arrangement of groups at the an ...
unit 4 revision checklist - A
... a) Derive an expression for the equilibrium constant Kc of a chemical reaction, and deduce its units b) Calculate the equilibrium constant of a reaction from equilibrium concentration data and vice versa c) Calculate the equilibrium constant of a reaction from initial concentration data and some inf ...
... a) Derive an expression for the equilibrium constant Kc of a chemical reaction, and deduce its units b) Calculate the equilibrium constant of a reaction from equilibrium concentration data and vice versa c) Calculate the equilibrium constant of a reaction from initial concentration data and some inf ...
chemical reaction
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and iodine. ...
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and iodine. ...
1 - PetyaPisanScienceAQ
... 1) Add about 0.5 g of sodium hydrogen carbonate to a test tube. Clamp this test tube on a right angle to the retort stand so that the sodium hydrogen carbonate is spread horizontally along the side of the tube. Heat the sodium hydrogen carbonate GENTLY for 2 minutes. Be sure not to heat the rubber o ...
... 1) Add about 0.5 g of sodium hydrogen carbonate to a test tube. Clamp this test tube on a right angle to the retort stand so that the sodium hydrogen carbonate is spread horizontally along the side of the tube. Heat the sodium hydrogen carbonate GENTLY for 2 minutes. Be sure not to heat the rubber o ...
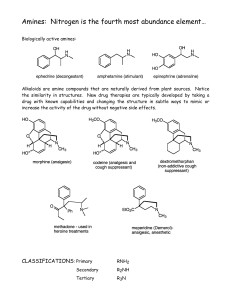
Amines: Nitrogen is the fourth most abundance element…
... Now you are left with naphthalene and benzoic acid. How would you separate these two compounds? Consider adding a base this time, such as sodium hydroxide in water. What happens this time? Nothing happens to naphthalene again, since it has no functional groups that react with base. Benzoic acid, tho ...
... Now you are left with naphthalene and benzoic acid. How would you separate these two compounds? Consider adding a base this time, such as sodium hydroxide in water. What happens this time? Nothing happens to naphthalene again, since it has no functional groups that react with base. Benzoic acid, tho ...
organic chemistry
... No more than one –OH group can be attached to any one carbon The carbon to which the –OH group is attached must have all single bonds Alcohols are not bases (do not ionize in water) Name: hydrocarbon name, replace the final –e with –ol ...
... No more than one –OH group can be attached to any one carbon The carbon to which the –OH group is attached must have all single bonds Alcohols are not bases (do not ionize in water) Name: hydrocarbon name, replace the final –e with –ol ...
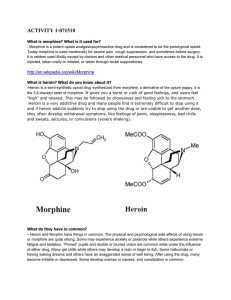
activity 1-071510 - ids
... Heroin addiction is a common problem in the United States and an issue that has not decreased in severity over the last decade. Morphine is less common, but just as serious of an issue. Most evolve into heroin addicts because the drug is so much stronger and easier to come by. Both morphine addicti ...
... Heroin addiction is a common problem in the United States and an issue that has not decreased in severity over the last decade. Morphine is less common, but just as serious of an issue. Most evolve into heroin addicts because the drug is so much stronger and easier to come by. Both morphine addicti ...
Hydrocarbon - TeacherWeb
... B. Number the ring, starting from one of the branches. Find the numbering that gives the lowest possible set of numbers for the branches. C. Name the substituents. D. Add the prefix to show the number of groups ...
... B. Number the ring, starting from one of the branches. Find the numbering that gives the lowest possible set of numbers for the branches. C. Name the substituents. D. Add the prefix to show the number of groups ...
Modules 261 12th edition
... How to test for Chirality: Planes of symmetry Naming Enantiomers: The R, S –System How to Assign (R) and (S) Configurations Properties of Enantiomers: Optical Activity - specific rotation - Plane polarized light - The polarimeter Racemic forms - Racemic forms and Enantiomeric Excess The Synthesis of ...
... How to test for Chirality: Planes of symmetry Naming Enantiomers: The R, S –System How to Assign (R) and (S) Configurations Properties of Enantiomers: Optical Activity - specific rotation - Plane polarized light - The polarimeter Racemic forms - Racemic forms and Enantiomeric Excess The Synthesis of ...
` United States
... 'It has ‘been known for many years that steroidal ketones 20 one-ZO-cyanohydrin preparation (paper presented to ‘be may ‘be converted into their 3-enol ethers or 3-cyclic ketals, by re?uxing the ketone and a mono- or polyhydric alcohol in benzene, in the presence of an acid catalyst, the ...
... 'It has ‘been known for many years that steroidal ketones 20 one-ZO-cyanohydrin preparation (paper presented to ‘be may ‘be converted into their 3-enol ethers or 3-cyclic ketals, by re?uxing the ketone and a mono- or polyhydric alcohol in benzene, in the presence of an acid catalyst, the ...
chemical reaction
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and ...
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and ...
Organic Chemistry - Moorpark College
... direction but you must end up with the lowest possible numbers. If only one substituent is connected in a cycloalkane there is no need to number the carbon 1 8) Also, for cycloalkanes there is a possible for stereoisomers since the ring structure will not permit free rotation. Stereoisomers may be l ...
... direction but you must end up with the lowest possible numbers. If only one substituent is connected in a cycloalkane there is no need to number the carbon 1 8) Also, for cycloalkanes there is a possible for stereoisomers since the ring structure will not permit free rotation. Stereoisomers may be l ...
Experiment 11 CHEMICAL REACTIONS
... In a combustion reaction an organic compound reacts with oxygen to produce CO 2 and water. An organic compound will have carbon and hydrogen in its formula, and possibly oxygen or other nonmetals. ...
... In a combustion reaction an organic compound reacts with oxygen to produce CO 2 and water. An organic compound will have carbon and hydrogen in its formula, and possibly oxygen or other nonmetals. ...
Alcohols/Wade
... 3. Draw the organic products you would expect to isolate from the following reactions after hydrolysis. a) . ...
... 3. Draw the organic products you would expect to isolate from the following reactions after hydrolysis. a) . ...
Unit 2A Organic Chem. Intro
... Work with a partner- SHARE THE COMPUTERS!! Get a laptop/computer and Dehydration Synthesis Gizmo Worksheet Login with student ID # and password you’ve created If you haven’t logged into a school computer this year use your student ID # ...
... Work with a partner- SHARE THE COMPUTERS!! Get a laptop/computer and Dehydration Synthesis Gizmo Worksheet Login with student ID # and password you’ve created If you haven’t logged into a school computer this year use your student ID # ...
Exercise #5_Chpt 2
... 1. In an exothermic reaction, chlorine reacts with 2.02 g of hydrogen to form 72.926 g of chlorine gas. How many grams of chlorine reacted with hydrogen? 2. Sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide . In sulfur dioxide, 32.06 g of sulfur are combined with 32.00 g of ...
... 1. In an exothermic reaction, chlorine reacts with 2.02 g of hydrogen to form 72.926 g of chlorine gas. How many grams of chlorine reacted with hydrogen? 2. Sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide . In sulfur dioxide, 32.06 g of sulfur are combined with 32.00 g of ...
42nd INTERNATIONAL CHEMISTRY OLYMPIAD
... The first titration was used to determine the proportion of copper ions in the complex. In this titration, the complex was reacted with EDTA solution. EDTA reacts with copper ions according to the equation: Cu2+(from the complex) + EDTA4–(aq) → [CuEDTA]2– (aq) The end-point of this titration was det ...
... The first titration was used to determine the proportion of copper ions in the complex. In this titration, the complex was reacted with EDTA solution. EDTA reacts with copper ions according to the equation: Cu2+(from the complex) + EDTA4–(aq) → [CuEDTA]2– (aq) The end-point of this titration was det ...
Microsoft Word format
... Students learn to differentiate the physical and chemical properties of substances, classify processes as physical or chemical changes, and learn that mass is conserved in chemical reactions. The observations include separation of iron and sulfur with a magnet, separation of sand and salt by dissolu ...
... Students learn to differentiate the physical and chemical properties of substances, classify processes as physical or chemical changes, and learn that mass is conserved in chemical reactions. The observations include separation of iron and sulfur with a magnet, separation of sand and salt by dissolu ...
Conservation of Energy in chemical reactions, Hess`s Law
... There are many chemical reactions that are difficult to study directly because the energy produced is very high, or because the reactants are difficult to obtain or handle. Hess’s Law (named after the scientist who proposed it) helps us to calculate H for a reaction by using data from other reactio ...
... There are many chemical reactions that are difficult to study directly because the energy produced is very high, or because the reactants are difficult to obtain or handle. Hess’s Law (named after the scientist who proposed it) helps us to calculate H for a reaction by using data from other reactio ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.