EKSIKA JOINT EVALUATION TEST. Kenya Certificate
... Write your name and index number in the spaces provided above. Sign and write the date of examination in the spaces provided above. Answer ALL questions in the spaces provided above. ...
... Write your name and index number in the spaces provided above. Sign and write the date of examination in the spaces provided above. Answer ALL questions in the spaces provided above. ...
the ap chemistry summer assignment
... Welcome to AP Chemistry! You already have a background in chemistry from your general chemistry class, but AP Chemistry is very different. Rather than memorizing how to do particular types of problems, you must really understand the chemistry and be able to apply it to different kinds of problems. A ...
... Welcome to AP Chemistry! You already have a background in chemistry from your general chemistry class, but AP Chemistry is very different. Rather than memorizing how to do particular types of problems, you must really understand the chemistry and be able to apply it to different kinds of problems. A ...
Pre-Lab Questions - Dr. Hornbuckle`s Home Page
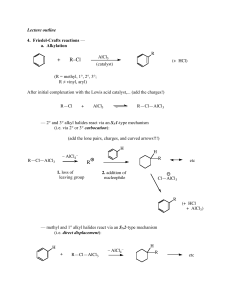
... 5) Using the reference book by J. March, give one method (brief description) which can be used to prepare compounds containing each of the following functional groups. All functional groups should be drawn in expanded form. Include the page number in the March textbook as a reference. (14 points) I) ...
... 5) Using the reference book by J. March, give one method (brief description) which can be used to prepare compounds containing each of the following functional groups. All functional groups should be drawn in expanded form. Include the page number in the March textbook as a reference. (14 points) I) ...
2 - DrChoChemistryWebSite
... We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We can’t remember them all, but luckily they will fall into several categories. We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
The Chemicals of Life Properties of Organic Compounds • Organic
... A carbon chain that contains one or more double bonds is called an alkene and the names end in “ene” and use a number to specify where the double bond is located, like 1-butene A carbon chain that contains one or more triple bonds is called an alkyne and the names end in “yne”, like 2-pentyne Hy ...
... A carbon chain that contains one or more double bonds is called an alkene and the names end in “ene” and use a number to specify where the double bond is located, like 1-butene A carbon chain that contains one or more triple bonds is called an alkyne and the names end in “yne”, like 2-pentyne Hy ...
Lecture 5: Biotrans_detox_biodegrade_lecture
... 5.) Phase I biotransformations (a.) occur mostly in the liver (b.) mediated by MFOs called cytochromes P450 (c.) occur in endoplasmic reticulum (liver microsomes) (d.) mechanism (e.) very broad range of substrates accomodated -- aliphatic hydroxylation -- aromatic hydroxylation -- aliphatic epoxida ...
... 5.) Phase I biotransformations (a.) occur mostly in the liver (b.) mediated by MFOs called cytochromes P450 (c.) occur in endoplasmic reticulum (liver microsomes) (d.) mechanism (e.) very broad range of substrates accomodated -- aliphatic hydroxylation -- aromatic hydroxylation -- aliphatic epoxida ...
File
... • Back bonding is significant in fisher and schrock carbenes but not with nucleophilic carbenes • For electron counting all carbenes donate two electrons • For oxidation state: • Fisher / Shrock carbenes (alylidenes) are dianionic (-2) • Nucleophillic carbenes are neutral (phosphines) ...
... • Back bonding is significant in fisher and schrock carbenes but not with nucleophilic carbenes • For electron counting all carbenes donate two electrons • For oxidation state: • Fisher / Shrock carbenes (alylidenes) are dianionic (-2) • Nucleophillic carbenes are neutral (phosphines) ...
fo-Balancing Chemical Notes
... CH3CH2OH + O2 ----> CO2 + H2O In this reaction, all of the compounds have the correct formulas. The next step is to select the 'simplest' element. Either carbon (C) or hydrogen (H) could be used. For this example, we will select carbon. Following step #3, we change the coefficients in front of ethan ...
... CH3CH2OH + O2 ----> CO2 + H2O In this reaction, all of the compounds have the correct formulas. The next step is to select the 'simplest' element. Either carbon (C) or hydrogen (H) could be used. For this example, we will select carbon. Following step #3, we change the coefficients in front of ethan ...
Question paper - Unit A173/02 - Module C7 - Higher tier
... speeds up other reactions of the esters as well as this reaction ...
... speeds up other reactions of the esters as well as this reaction ...
Chemdraw B&W
... NH3, primary (RNH2) and secondary amines (R2NH) • The reaction with tertiary amines (R3N) gives an unstable species that cannot be isolated • HCl is neutralized by the amine or an added base ...
... NH3, primary (RNH2) and secondary amines (R2NH) • The reaction with tertiary amines (R3N) gives an unstable species that cannot be isolated • HCl is neutralized by the amine or an added base ...
Determining the Structure of Primary, Secondary, and Tertiary Alcohols
... Question: What does it mean for an alcohol to be primary, secondary, or tertiary? Procedure: Part 1 1. Place 2 mL of 0.01 mol/L KMnO4 in a test tube. 2. Add 2 mL of the alcohol labeled as “primary” and 1 mL of water. Place a stopper on the test tube and shake. 3. Record your observations at one minu ...
... Question: What does it mean for an alcohol to be primary, secondary, or tertiary? Procedure: Part 1 1. Place 2 mL of 0.01 mol/L KMnO4 in a test tube. 2. Add 2 mL of the alcohol labeled as “primary” and 1 mL of water. Place a stopper on the test tube and shake. 3. Record your observations at one minu ...
Wizard Test Maker
... 32. Which pH value indicates the most basic 35. The water solution of which of the solution? following substances is the best conductor of electricity? A 3 A KCl B 7 B C6H12O6 C 8 C CO2 D 11 D CO 33. When ethylene glycol (an antifreeze) is added to water, what happens to the boiling point of the wat ...
... 32. Which pH value indicates the most basic 35. The water solution of which of the solution? following substances is the best conductor of electricity? A 3 A KCl B 7 B C6H12O6 C 8 C CO2 D 11 D CO 33. When ethylene glycol (an antifreeze) is added to water, what happens to the boiling point of the wat ...
1.0 basic concepts
... • If you look at the reactants in (a), you’ll notice that the metal has no oxygen present. • This means that water, H2O cannot be formed, therefore H2 is the product • If you look at the reactants in (a) – (d), you’ll notice that the metal has oxygen present. • This means that water, H2O can be form ...
... • If you look at the reactants in (a), you’ll notice that the metal has no oxygen present. • This means that water, H2O cannot be formed, therefore H2 is the product • If you look at the reactants in (a) – (d), you’ll notice that the metal has oxygen present. • This means that water, H2O can be form ...
Chapter 27. Biomolecules: Lipids
... The hydrocarbon tail is nonpolar and dissolves in grease and oil Soaps enable grease to be dissolved into water ...
... The hydrocarbon tail is nonpolar and dissolves in grease and oil Soaps enable grease to be dissolved into water ...
AQA_GCSE_Chemistry_Higher_Unit_2_Notes
... In these substances, strong covalent bonds join atoms together in large numbers to make giant structures. Sand (silicon dioxide) is one example, diamond and graphite (both forms of carbon) are others are others.Because the bonds between all the atoms are very strong: 1) They have very high melting p ...
... In these substances, strong covalent bonds join atoms together in large numbers to make giant structures. Sand (silicon dioxide) is one example, diamond and graphite (both forms of carbon) are others are others.Because the bonds between all the atoms are very strong: 1) They have very high melting p ...
CLASS X carbon and its compound
... A reaction which proceeds with the breaking of double or triple covalent bonds in organic compounds, so as to form new organic compounds having single covalent bond, is called addition reaction. The conversion of unsaturated vegetable oil into saturated vegetable oil by the absorption of hydrogen in ...
... A reaction which proceeds with the breaking of double or triple covalent bonds in organic compounds, so as to form new organic compounds having single covalent bond, is called addition reaction. The conversion of unsaturated vegetable oil into saturated vegetable oil by the absorption of hydrogen in ...
Catalysis Web Pages for Pre-University
... Titanium (III) chloride, (TiCl3), titanium (IV) chloride (TiCl4 ) and aluminium triethyl (Al2(CH3CH2)3 ) are Ziegler-Natta catalysts and are used in the polymerisation of ethene, C2H4, (an alkene) to high density poly(ethane). The pressure used is 50-75 oC. Poly(ethene), more commonly called polythe ...
... Titanium (III) chloride, (TiCl3), titanium (IV) chloride (TiCl4 ) and aluminium triethyl (Al2(CH3CH2)3 ) are Ziegler-Natta catalysts and are used in the polymerisation of ethene, C2H4, (an alkene) to high density poly(ethane). The pressure used is 50-75 oC. Poly(ethene), more commonly called polythe ...
Click to download. - Life Learning Cloud
... AN ION is an atom or group of atoms with an electrical charge (+ or -). Metal compounds such as sodium chloride or copper sulphate contain ions. Sodium chloride is made of Na+ and Cl- ions Copper Sulphate is made of Cu2+ and SO42- ions Note that metals form positive ions while non-metals form negati ...
... AN ION is an atom or group of atoms with an electrical charge (+ or -). Metal compounds such as sodium chloride or copper sulphate contain ions. Sodium chloride is made of Na+ and Cl- ions Copper Sulphate is made of Cu2+ and SO42- ions Note that metals form positive ions while non-metals form negati ...
... Based on the results and literature data, it was proposed that Acy-I catalyzes the synthesis (rather than hydrolysis) of hippurate, which is formed as a detoxification product of aromatic compounds. Regarding the application of this enzyme in organic chemistry, the acylase from Aspergillus spp was u ...
Catalytic, Enantioselective Alkylation of r
... although slight uncatalyzed reaction in THF solution at -50 °C between 1a and 2a was initially a cause for concern. Surprisingly, however, slow addition of 1.1 equiv enol silane 2a over the course of 2 h into a solution of the R-imino ester 1a containing 10 mol % of (R)-BINAP-AgSbF613 (3a) at -80 °C ...
... although slight uncatalyzed reaction in THF solution at -50 °C between 1a and 2a was initially a cause for concern. Surprisingly, however, slow addition of 1.1 equiv enol silane 2a over the course of 2 h into a solution of the R-imino ester 1a containing 10 mol % of (R)-BINAP-AgSbF613 (3a) at -80 °C ...
Chemistry Name: LeChâtlier`s Principle Date: Chemical Equilibrium
... Sulfur dioxide is added to the system. left ...
... Sulfur dioxide is added to the system. left ...
1. Explain electrophile and nucleophile. 2. Explain
... 69. Draw the resonance structures for the following compounds. Show the electron shift using curved arrow rotation. (i) C6H5OH (2) C6H5NO2 (3) C6H5C+H2 (4) CH3CH=CHCHO (5) CH3CH=CHC+H2 (6)C6H5CHO (7) CH2=CHOCH3. 70. Write chemical equations describing the acid catalyzed dehydration of ethanol. 71. W ...
... 69. Draw the resonance structures for the following compounds. Show the electron shift using curved arrow rotation. (i) C6H5OH (2) C6H5NO2 (3) C6H5C+H2 (4) CH3CH=CHCHO (5) CH3CH=CHC+H2 (6)C6H5CHO (7) CH2=CHOCH3. 70. Write chemical equations describing the acid catalyzed dehydration of ethanol. 71. W ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.