1. You should review balancing equations and identifying types of
... 1. You should review balancing equations and identifying types of reactions from the worksheets. In addition you should be able to write balanced chemical equations for reactions. Try to write, balance, and identify the types of the following reactions: a. the decomposition of ammonium nitrate to ni ...
... 1. You should review balancing equations and identifying types of reactions from the worksheets. In addition you should be able to write balanced chemical equations for reactions. Try to write, balance, and identify the types of the following reactions: a. the decomposition of ammonium nitrate to ni ...
Document
... Directions: In the following activity, you are going to build different types of simple organic molecules. After each step you should return back to the original molecule, before proceeding to the next arrangement. Follow the directions to this lab very carefully. For each direction that cannot be f ...
... Directions: In the following activity, you are going to build different types of simple organic molecules. After each step you should return back to the original molecule, before proceeding to the next arrangement. Follow the directions to this lab very carefully. For each direction that cannot be f ...
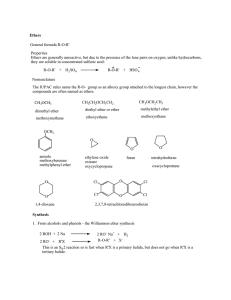
How to Name Alcohols
... bonds, in the numbering of the parent chain. If there is a choice in numbering after these rules are followed, the parent chain is numbered to give the double bonds and then substituents the lowest number. ...
... bonds, in the numbering of the parent chain. If there is a choice in numbering after these rules are followed, the parent chain is numbered to give the double bonds and then substituents the lowest number. ...
Preparation of Aldehydes and Ketones
... NaBH4 and LiAlH4 reduce carbonyl groups but not carbon-carbon double bonds: ...
... NaBH4 and LiAlH4 reduce carbonyl groups but not carbon-carbon double bonds: ...
Part One: Ions in Aqueous Solution A. Electrolytes and Non
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is ...
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is ...
Ir-catalysed formation of C− F bonds. From allylic alcohols to α
... Scheme 3 Proposed catalytic cycle. ...
... Scheme 3 Proposed catalytic cycle. ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
Keq Assignment
... e) solid hydrogen cyanide dissolves to produce hydrogen ions and cyanide ions in solution. ...
... e) solid hydrogen cyanide dissolves to produce hydrogen ions and cyanide ions in solution. ...
CHEMISTRY OF CARBOHYDRATES
... change when it is reduced The intensity of the colour change is proportional to the concentration of reducing ...
... change when it is reduced The intensity of the colour change is proportional to the concentration of reducing ...
CHEMISTRY OF CARBOHYDRATES
... change when it is reduced The intensity of the colour change is proportional to the concentration of reducing ...
... change when it is reduced The intensity of the colour change is proportional to the concentration of reducing ...
Example
... Very strong C═O stretch around 1710 cm-1 for ketones and 1725 cm-1 for simple aldehydes Additional C—H stretches for aldehyde: Two absorptions at 2710 cm-1 and 2810 cm-1 © 2013 Pearson Education, Inc. ...
... Very strong C═O stretch around 1710 cm-1 for ketones and 1725 cm-1 for simple aldehydes Additional C—H stretches for aldehyde: Two absorptions at 2710 cm-1 and 2810 cm-1 © 2013 Pearson Education, Inc. ...
Biosynthesis of 2-hydroxyisobutyric acid (2
... significantly. This tertiary carbon atom-containing C4 carboxylic acid is currently not a high-volume product of the chemical industry but only a specialty chemical. It is used to some extend as a pharmaceutical intermediate and also as a complex-forming agent for lanthanide and actinide heavy metal ...
... significantly. This tertiary carbon atom-containing C4 carboxylic acid is currently not a high-volume product of the chemical industry but only a specialty chemical. It is used to some extend as a pharmaceutical intermediate and also as a complex-forming agent for lanthanide and actinide heavy metal ...
Solution - gearju.com
... A 0.5662-g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of AgNO3. If 1.0882 g of AgCl precipitate forms, what is the percent by mass of Cl in the original compound? ...
... A 0.5662-g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of AgNO3. If 1.0882 g of AgCl precipitate forms, what is the percent by mass of Cl in the original compound? ...
GCE Chemistry Question Paper Unit 05 - Energetics, Redox
... outside the box around each page or on blank pages. l All working must be shown. l Do all rough work in this book. Cross through any work you do not want to be marked. ...
... outside the box around each page or on blank pages. l All working must be shown. l Do all rough work in this book. Cross through any work you do not want to be marked. ...
Scope and Limitations - Organic Reactions Wiki
... amounts of transition metal due to the inability of the resulting osmate(VI) ester to undergo either direct hydrolysis or in situ oxidation to a more easily hydrolyzed Os(VIII) species. By switching to monodentate amines such as quinuclidine, introduced as its Noxide (QNO), the reactivity and hydrog ...
... amounts of transition metal due to the inability of the resulting osmate(VI) ester to undergo either direct hydrolysis or in situ oxidation to a more easily hydrolyzed Os(VIII) species. By switching to monodentate amines such as quinuclidine, introduced as its Noxide (QNO), the reactivity and hydrog ...
My title - Revista Mexicana de Ingeniería Química
... trials were conducted in duplicate. Hexadecane was used as an internal standard. The second strategy was carried out following the protocol described for the former, except that in the second approach we employed MeOH, evaluating the deactivation of CALB as FAME content. The third strategy was perfo ...
... trials were conducted in duplicate. Hexadecane was used as an internal standard. The second strategy was carried out following the protocol described for the former, except that in the second approach we employed MeOH, evaluating the deactivation of CALB as FAME content. The third strategy was perfo ...
Experimental Study of Closed System in the Chlorine Dioxide
... citric acid solution. Sulfuric acid: 0.05 mol/L. All other chemicals were the highest purity commercially available and were used as received. 2.2. Methods. The reaction was started by injecting a small volume of one of the reactants into a mixture containing the other components in a spectrophotome ...
... citric acid solution. Sulfuric acid: 0.05 mol/L. All other chemicals were the highest purity commercially available and were used as received. 2.2. Methods. The reaction was started by injecting a small volume of one of the reactants into a mixture containing the other components in a spectrophotome ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.