Catalysts 1
... solvent-free conditions [34]. There have also been reports on the acylation of alcohols using acetic anhydride, catalyzed by silica gel supported Ce(SO4)2, Ti(SO4)2, Fe2(SO4)3, and NaHSO4 [35]. Although the majority of these methods ensure good results, there is still a great need for simple, mild, ...
... solvent-free conditions [34]. There have also been reports on the acylation of alcohols using acetic anhydride, catalyzed by silica gel supported Ce(SO4)2, Ti(SO4)2, Fe2(SO4)3, and NaHSO4 [35]. Although the majority of these methods ensure good results, there is still a great need for simple, mild, ...
Here is the Original File - University of New Hampshire
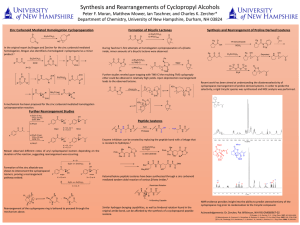
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
Substitution Rxns-a-Sn2-12-quesx
... arrangement of bonds in product is opposite to that of reactant ...
... arrangement of bonds in product is opposite to that of reactant ...
Williamson Ether Synthesis
... Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxid ...
... Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxid ...
102 Lecture Ch17
... (except PCC, which forms aldehydes) • Aldehydes can be oxidized to carboxylic acids with most oxidizing agents, such as Tollens’reagent (AgNO3/NH3) - alcohols do not react with Tollens CrO3 OH ...
... (except PCC, which forms aldehydes) • Aldehydes can be oxidized to carboxylic acids with most oxidizing agents, such as Tollens’reagent (AgNO3/NH3) - alcohols do not react with Tollens CrO3 OH ...
View/Open
... (Section 11.2) in some respects. For example, they are able to form strong intermolecular hydrogen bonds, and therefore have higher boiling points than hydrocarbons of the same molecular weight. Phenol (bp 182 8C) has a boiling point more than 70 8C higher than toluene (bp 110.6 8C), even though th ...
... (Section 11.2) in some respects. For example, they are able to form strong intermolecular hydrogen bonds, and therefore have higher boiling points than hydrocarbons of the same molecular weight. Phenol (bp 182 8C) has a boiling point more than 70 8C higher than toluene (bp 110.6 8C), even though th ...
Chemistry Notes - The Bored of Studies Community
... A stoichiometric mixture of hydrogen and nitrogen is used because, as ammonia is formed and condensed out, left-over reactants can be recycled through the process (with some fresh reactant mixture added) without any build-up of one reactant over the other. An important factor in designing an industr ...
... A stoichiometric mixture of hydrogen and nitrogen is used because, as ammonia is formed and condensed out, left-over reactants can be recycled through the process (with some fresh reactant mixture added) without any build-up of one reactant over the other. An important factor in designing an industr ...
Derivatives of Carboxylic Acids
... salicylate ester of acetic acid. • Salicylic acid is a bifunctional molecule (acid and phenol) which is itself a disinfectant. • Aspirin comes from the willow bark which was used in the Middle Ages by Jesuit missionaries. ...
... salicylate ester of acetic acid. • Salicylic acid is a bifunctional molecule (acid and phenol) which is itself a disinfectant. • Aspirin comes from the willow bark which was used in the Middle Ages by Jesuit missionaries. ...
Assistant Professor Chemistry, Class-2, Advt No. 84/2016
... Which of the following species does not have metal-metal bond (A) Fe2(CO)9 ...
... Which of the following species does not have metal-metal bond (A) Fe2(CO)9 ...
EXPERIMENT 5 (Organic Chemistry II) Pahlavan/Cherif
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
Dehydration of Alcohols - Dehydration of Cyclohexanol
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
... There are four basic types of chemical reactions in organic chemistry: combination, elimination, substitution, and rearrangement. The dehydration of alcohols to give alkenes is an important transformation and is an example of elimination reaction. Strong mineral acids such as sulfuric and phosphoric ...
File - Mr Weng`s IB Chemistry
... • A thermosetting polymer is a prepolymer in a soft solid or viscous state that changes irreversibly into a hardened thermoset by curing. • Elastomers are flexible and can be deformed under force but will return to nearly their original shape once the stress is released. • High density polyethene (H ...
... • A thermosetting polymer is a prepolymer in a soft solid or viscous state that changes irreversibly into a hardened thermoset by curing. • Elastomers are flexible and can be deformed under force but will return to nearly their original shape once the stress is released. • High density polyethene (H ...
Pyro without Fire: Synthesis, Structure, and Reactivity of a Dimeric
... reaction in mild conditions. Fundamental reactivity studies conducted with 2 show catalytic activity, although each metal ion in the starting complex is coordinatively saturated. The catalytic aerobic oxidation of benzyl alcohol (ba) was used as a benchmark reaction because this would allow for faci ...
... reaction in mild conditions. Fundamental reactivity studies conducted with 2 show catalytic activity, although each metal ion in the starting complex is coordinatively saturated. The catalytic aerobic oxidation of benzyl alcohol (ba) was used as a benchmark reaction because this would allow for faci ...
Journal of Physical and Chemical Reference Data
... actually or hypothetically react with each other in an astronomical number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These r ...
... actually or hypothetically react with each other in an astronomical number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These r ...
4.4 Formation of Esters from Carboxylic Acids and Alcohols
... process. Note that a water molecule is removed in the process of forming the ester from the carboxylic acid and the alcohol. The water comes from removing an OH group on the carboxylic acid and combining it with a H. One can show this using “lasso” chemistry as shown ...
... process. Note that a water molecule is removed in the process of forming the ester from the carboxylic acid and the alcohol. The water comes from removing an OH group on the carboxylic acid and combining it with a H. One can show this using “lasso” chemistry as shown ...
University of Groningen Magnesium and zinc hydride
... In the second chapter of this thesis, detailed studies on the properties of these multinuclear complexes are presented. Whereas these compounds are very stable in solution, decomposition in the solid state shows reductive elimination of the incorporated hydride ligands as hydrogen and a clear tenden ...
... In the second chapter of this thesis, detailed studies on the properties of these multinuclear complexes are presented. Whereas these compounds are very stable in solution, decomposition in the solid state shows reductive elimination of the incorporated hydride ligands as hydrogen and a clear tenden ...
Chapter 12 and 13 Notes
... Contain the –OH group as in R-OH. Nomenclature of alcohols: 1. Find the longest chain of C’s, which also includes the –OH group. Change the –e at the end of the usual base name to –ol. 2. Number the chain so that the –OH group has the lowest number ALWAYS!!! 3. Name any other substituents using ...
... Contain the –OH group as in R-OH. Nomenclature of alcohols: 1. Find the longest chain of C’s, which also includes the –OH group. Change the –e at the end of the usual base name to –ol. 2. Number the chain so that the –OH group has the lowest number ALWAYS!!! 3. Name any other substituents using ...
Polyesters are condensation polymers.
... Note that sometimes other molecules (HCl for eg) are lost in other condensation reactions. It is the elimination of a molecule which makes it a condensation reaction, not the loss of water. ...
... Note that sometimes other molecules (HCl for eg) are lost in other condensation reactions. It is the elimination of a molecule which makes it a condensation reaction, not the loss of water. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.