Naming Aldehydes & Ketones
... IUPAC Rules for Naming Ketones 3. If the chain is longer than four carbons, it is numbered so that the carbonyl group has the smallest number possible; this number is prefixed to the parent name of the ketone. 4. Other groups attached to the parent chain are named and numbered as we have done befor ...
... IUPAC Rules for Naming Ketones 3. If the chain is longer than four carbons, it is numbered so that the carbonyl group has the smallest number possible; this number is prefixed to the parent name of the ketone. 4. Other groups attached to the parent chain are named and numbered as we have done befor ...
organic chem notes
... common methods of making nylon for fiber applications. In one approach, molecules with an acid (COOH) group on each end are reacted with molecules containing amine (NH2) groups on each end. The resulting nylon is named on the basis of the number of carbon atoms separating the two acid groups and the ...
... common methods of making nylon for fiber applications. In one approach, molecules with an acid (COOH) group on each end are reacted with molecules containing amine (NH2) groups on each end. The resulting nylon is named on the basis of the number of carbon atoms separating the two acid groups and the ...
Exam 3 Review Key
... SH, OH, and N sites (3 on BAL, 4 on DMSA, 6 on EDTA) NOTE: C=O oxygens are not Lewis bases and will not act as ligand sites b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Conside ...
... SH, OH, and N sites (3 on BAL, 4 on DMSA, 6 on EDTA) NOTE: C=O oxygens are not Lewis bases and will not act as ligand sites b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Conside ...
e-nomenclature-of-coordination-compounds-take-home-2
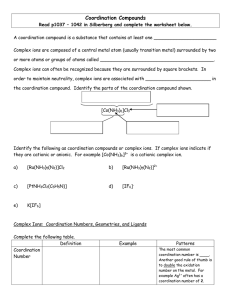
... Coordination Compounds Read p1037 – 1042 in Silberberg and complete the worksheet below. A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of ...
... Coordination Compounds Read p1037 – 1042 in Silberberg and complete the worksheet below. A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of ...
PowerPoint - Organic Chemistry
... • All of these have a plant origin • All of these rely on the “fixing” of C from CO2 • Synthetic organic compounds are derived from fossil fuels or plant material ...
... • All of these have a plant origin • All of these rely on the “fixing” of C from CO2 • Synthetic organic compounds are derived from fossil fuels or plant material ...
spring semester review
... 74. How many grams of Ca metal could be produced by the electrolysis of molten CaBr 2 using a current of 30.0 amp for 10.0 hours? a) 22.4 g b) 448 g c) 0.0622 g d) 224 g 75. How long will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps? a) 5.77 minu ...
... 74. How many grams of Ca metal could be produced by the electrolysis of molten CaBr 2 using a current of 30.0 amp for 10.0 hours? a) 22.4 g b) 448 g c) 0.0622 g d) 224 g 75. How long will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps? a) 5.77 minu ...
File
... CH2OHCH2OH = 1,2-ethanediol or ethylene glycol 2) Alcohols which have 3 OH goups are called triols CH2OHCHOHCH2OH = 1,2, 3-propanetriol or glycerol or glycerin ...
... CH2OHCH2OH = 1,2-ethanediol or ethylene glycol 2) Alcohols which have 3 OH goups are called triols CH2OHCHOHCH2OH = 1,2, 3-propanetriol or glycerol or glycerin ...
final-H-2006-07-v1
... c. there is no relationship between the temperature and the solubility 80. Many laboratory preparations of solutions call for stirring the solvent while adding the solute. Which of the following is always an effect of this procedure? a. It decreases the reactivity of the solute. b. It decreases the ...
... c. there is no relationship between the temperature and the solubility 80. Many laboratory preparations of solutions call for stirring the solvent while adding the solute. Which of the following is always an effect of this procedure? a. It decreases the reactivity of the solute. b. It decreases the ...
IA Practical Report Properties of Alkanes and Alkenes
... b) What kind of reaction occurs when Br2 and cyclohexene are mixed? c) Draw the reaction of ethene and Br2 (aq). Draw the reaction that cyclohexene underwent. Why did the colour change during the reaction? 7. KMnO4 is known as an “oxidizing agent” because it adds oxygen to other compounds. In this l ...
... b) What kind of reaction occurs when Br2 and cyclohexene are mixed? c) Draw the reaction of ethene and Br2 (aq). Draw the reaction that cyclohexene underwent. Why did the colour change during the reaction? 7. KMnO4 is known as an “oxidizing agent” because it adds oxygen to other compounds. In this l ...
1. Absorption of what type electromagnetic radiation results in
... How many nodes, other than the node coincident with the molecular plane, are found in the highest energy π MO of 1,3-butadiene? A. B. C. D. E. ...
... How many nodes, other than the node coincident with the molecular plane, are found in the highest energy π MO of 1,3-butadiene? A. B. C. D. E. ...
F Practice Test #2 Solutions
... C) No reaction will occur. D) Both KNO3 and NiS precipitate from solution. E) KNO3 will precipitate from solution. 10. Which of the following statements concerning equilibrium is not true? A) The equilibrium constant is independent of temperature. B) The value of the equilibrium constant for a given ...
... C) No reaction will occur. D) Both KNO3 and NiS precipitate from solution. E) KNO3 will precipitate from solution. 10. Which of the following statements concerning equilibrium is not true? A) The equilibrium constant is independent of temperature. B) The value of the equilibrium constant for a given ...
Name: Date: Hour - Pointbiolabs.com
... ____ 20. The molecules of life are all carbon-based. _________________________ ____ 21. Carbon-carbon bonds can be single, double, or triple ionic bonds. _________________________ ____ 22. Lipids are important parts of biological membranes and waterproof coverings. _________________________ ____ 23. ...
... ____ 20. The molecules of life are all carbon-based. _________________________ ____ 21. Carbon-carbon bonds can be single, double, or triple ionic bonds. _________________________ ____ 22. Lipids are important parts of biological membranes and waterproof coverings. _________________________ ____ 23. ...
2008 Periodic Table of the Elements Instructions: Read carefully all
... Two identical gas containers containing N2 and O2 have the exact same number of moles, but the O2 is stored at 20°C while the N2 container is stored at 40°C. Which of the following statements is true? a) The molecules of the two gases have the same kinetic energy b) P(for N2) = 2 x P(for O2) c) The ...
... Two identical gas containers containing N2 and O2 have the exact same number of moles, but the O2 is stored at 20°C while the N2 container is stored at 40°C. Which of the following statements is true? a) The molecules of the two gases have the same kinetic energy b) P(for N2) = 2 x P(for O2) c) The ...
Unit 2: Carbon Compounds
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
... A fuel is a chemical which is burned to produce energy. Combustion is another word for burning. When a substance burns it reacts with oxygen. The chemical compounds which are found in oil and natural gas are mainly hydrocarbons. A hydrocarbon is a compound which contains hydrogen and carbo ...
Organic Functional Groups to know ASAP!
... *The following organic functional groups will be studied throughout the course of the year: naming, physical/chemical properties, reactions, mechanisms, & synthesis. *Your first assignment is to become familiar (memorize) the following information so that you can easily work with all material whethe ...
... *The following organic functional groups will be studied throughout the course of the year: naming, physical/chemical properties, reactions, mechanisms, & synthesis. *Your first assignment is to become familiar (memorize) the following information so that you can easily work with all material whethe ...
Metal complexes of di-phosphorus imines. An exploration of
... carried out in mixed water/solvent medium and the imine form trapped as a stable complex (11a)22. Careful hydrolysis with methanol in organic solvents also yields the imine in solution but we have not isolated these free imines. In solution, the imine converts over time to the phosphine oxide. When ...
... carried out in mixed water/solvent medium and the imine form trapped as a stable complex (11a)22. Careful hydrolysis with methanol in organic solvents also yields the imine in solution but we have not isolated these free imines. In solution, the imine converts over time to the phosphine oxide. When ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.