ch16 by dr. Dina
... formation of an equilibrium between the carbonyl compound and its hydrate The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is ster ...
... formation of an equilibrium between the carbonyl compound and its hydrate The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is ster ...
Abbreviated Chapter 17 Powerpoint
... • Reaction fails if benzene has a substituent that is more deactivating than halogens. • Rearrangements are possible. • The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. ...
... • Reaction fails if benzene has a substituent that is more deactivating than halogens. • Rearrangements are possible. • The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. ...
Study Guide 1 - Chemistry Teaching Resources
... acids. Which is the monomer and which is the polymer? ...
... acids. Which is the monomer and which is the polymer? ...
IUPAC nomenclature of organic chemistry
... Cycloalkanes and aromatic compounds can be treated as the main parent chain of the compound, in which case the position of substituents are numbered around the ring structure. For example, the three isomers of xylene CH3C6H4CH3, commonly the ortho-, meta-, and para- forms, are 1,2-dimethylbenzene, 1 ...
... Cycloalkanes and aromatic compounds can be treated as the main parent chain of the compound, in which case the position of substituents are numbered around the ring structure. For example, the three isomers of xylene CH3C6H4CH3, commonly the ortho-, meta-, and para- forms, are 1,2-dimethylbenzene, 1 ...
102 Lecture Ch15
... • Recall that aldehydes and ketones are formed by the oxidation of primary and secondary alcohols, respectively • Also recall that aldehydes are readily oxidized to carboxylic acids, but ketones are not • Tollens’ reagent (silver nitrate plus ammonia) can be used to distinguish between ketones and a ...
... • Recall that aldehydes and ketones are formed by the oxidation of primary and secondary alcohols, respectively • Also recall that aldehydes are readily oxidized to carboxylic acids, but ketones are not • Tollens’ reagent (silver nitrate plus ammonia) can be used to distinguish between ketones and a ...
AddCorrections(KKH) - Spiral
... homoallylarenes and 1-hexene, side-products were observed, formed from isomerisation of the C=C bond prior to reaction with the nucleophile. Four equivalents of olefin were used in all cases, although much of this could be recovered at the end of the reaction. A year later, the same group showed tha ...
... homoallylarenes and 1-hexene, side-products were observed, formed from isomerisation of the C=C bond prior to reaction with the nucleophile. Four equivalents of olefin were used in all cases, although much of this could be recovered at the end of the reaction. A year later, the same group showed tha ...
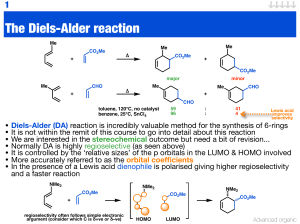
The Diels-Alder reaction
... • Another hetero-Diels-Alder reaction • It looks very similar to the previous reaction but... • It is believed that only one hydrogen bond activates the aldehyde • The other is used to form a rigid chiral environment for the reaction Advanced organic ...
... • Another hetero-Diels-Alder reaction • It looks very similar to the previous reaction but... • It is believed that only one hydrogen bond activates the aldehyde • The other is used to form a rigid chiral environment for the reaction Advanced organic ...
catalysis lecture
... There are 5 types of reactions (and their reverse) which, in combination, account for most homogeneous catalytic cycles involving hydrocarbons. 1. Ligand Coordination and Dissociation 2. Insertion and Elimination 3. Nucleophilic attack on coordinated ligands 4. Oxidation and Reduction 5. Oxidative a ...
... There are 5 types of reactions (and their reverse) which, in combination, account for most homogeneous catalytic cycles involving hydrocarbons. 1. Ligand Coordination and Dissociation 2. Insertion and Elimination 3. Nucleophilic attack on coordinated ligands 4. Oxidation and Reduction 5. Oxidative a ...
Lecture 18
... Epinephrine (adrenaline) and norepinephrine (noradrenaline) are released by the adrenal medulla during stressful situations They raise the blood glucose level and move blood to the muscles. The prefix nor in a drug name means there is one less —CH3 group on the nitrogen atom. Norepinephrine is used ...
... Epinephrine (adrenaline) and norepinephrine (noradrenaline) are released by the adrenal medulla during stressful situations They raise the blood glucose level and move blood to the muscles. The prefix nor in a drug name means there is one less —CH3 group on the nitrogen atom. Norepinephrine is used ...
Thiobenzoate Photochemistry
... While the elimination occurs with compounds possessing a -bond or heteroatom at the carbon, it fails with saturated hydrocarbons. The success of the reaction depends on the strength of the -C-H bond. For example, the quantum yields for the disappearance of Ph(C=S)OCH2CH2R are 0.46, 0.12 and 0.01 ...
... While the elimination occurs with compounds possessing a -bond or heteroatom at the carbon, it fails with saturated hydrocarbons. The success of the reaction depends on the strength of the -C-H bond. For example, the quantum yields for the disappearance of Ph(C=S)OCH2CH2R are 0.46, 0.12 and 0.01 ...
Support material for lesson planning – AS content
... The table on the following pages sets out suggested teaching times for the topics within the Chemistry A AS Level specification from 2015 (H032). This information can also be used in the context of teaching the Chemistry A A Level specification from 2015 (H432). Note that the timings are suggested o ...
... The table on the following pages sets out suggested teaching times for the topics within the Chemistry A AS Level specification from 2015 (H032). This information can also be used in the context of teaching the Chemistry A A Level specification from 2015 (H432). Note that the timings are suggested o ...
Origin of the Diastereoselection in the Indium
... phenyl group (anti/syn ) 1:4), which presumably destabilized the conformation A by the steric interactions with the ligands on indium. In summary, the In-mediated addition reaction of the haloallylic sulfones 1 to aldehydes 2 efficiently produces the homoallylic alcohols 3 containing a homoallylic s ...
... phenyl group (anti/syn ) 1:4), which presumably destabilized the conformation A by the steric interactions with the ligands on indium. In summary, the In-mediated addition reaction of the haloallylic sulfones 1 to aldehydes 2 efficiently produces the homoallylic alcohols 3 containing a homoallylic s ...
Ch 12 Alcohols and Thiols
... 2. Van der Waals – depending on Carbon chain length • Length of Carbon chain • C1-C2 gas, C3 -C10: liquid, C11 and higher: solids ...
... 2. Van der Waals – depending on Carbon chain length • Length of Carbon chain • C1-C2 gas, C3 -C10: liquid, C11 and higher: solids ...
Survival Organic Chemistry Part I: Molecular Models
... Use the space below, and on the next page, to draw the Lewis structure for each of the compounds and any structural isomers they may have. ...
... Use the space below, and on the next page, to draw the Lewis structure for each of the compounds and any structural isomers they may have. ...
File
... The nitro-group is an electron-withdrawing group. The presence of this group in the ortho position decreases the electron density in the O−H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitr ...
... The nitro-group is an electron-withdrawing group. The presence of this group in the ortho position decreases the electron density in the O−H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitr ...
Halogenoalkanes
... When methanal (CH2 O) is added, a primary alcohol with one extra carbon atom is formed. When other aldehydes are used (e.g. ethanal), secondary alcohols are formed. When ketones are used (e.g. pro panone), tertiary alcohols are formed. When CO2 is used, a carboxylic acid with one extra carbon is for ...
... When methanal (CH2 O) is added, a primary alcohol with one extra carbon atom is formed. When other aldehydes are used (e.g. ethanal), secondary alcohols are formed. When ketones are used (e.g. pro panone), tertiary alcohols are formed. When CO2 is used, a carboxylic acid with one extra carbon is for ...
aldehydes and ketones
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
File - chemistryworkshopjr
... CONCEPT DETAIL-Catalysis is the increase in the rate of a chemical reaction of one or more reactants due to the participation of an additional substance called a catalyst. Unlike other reagents in the chemical reaction, a catalyst is not consumed by the reaction. With a catalyst, less free energy is ...
... CONCEPT DETAIL-Catalysis is the increase in the rate of a chemical reaction of one or more reactants due to the participation of an additional substance called a catalyst. Unlike other reagents in the chemical reaction, a catalyst is not consumed by the reaction. With a catalyst, less free energy is ...
Reactions of Molecules with Oxygen
... Because alcohols are covalent compounds, they will usually not form ions when they lose electrons. But electrons will shift away from the atom that is oxidized. (You will learn more about this in Gr 12!) Two signs that an organic compound has undergone oxidation are: 1. The compound gains oxygen ato ...
... Because alcohols are covalent compounds, they will usually not form ions when they lose electrons. But electrons will shift away from the atom that is oxidized. (You will learn more about this in Gr 12!) Two signs that an organic compound has undergone oxidation are: 1. The compound gains oxygen ato ...
16.18 Summary
... Acid-catalyzed condensation of alcohols (Sections 15.7 and 16.5) Two molecules of an alcohol condense in the presence of an acid catalyst to yield a dialkyl ether and water. The reaction is limited to the synthesis of symmetrical ethers from primary alcohols. Williamson ether synthesis (Section 16. ...
... Acid-catalyzed condensation of alcohols (Sections 15.7 and 16.5) Two molecules of an alcohol condense in the presence of an acid catalyst to yield a dialkyl ether and water. The reaction is limited to the synthesis of symmetrical ethers from primary alcohols. Williamson ether synthesis (Section 16. ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.